Pentoxifylline
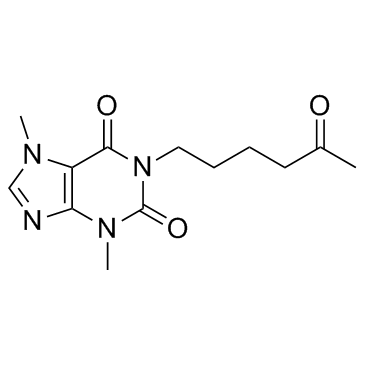
Pentoxifylline structure
|
Common Name | Pentoxifylline | ||
|---|---|---|---|---|
| CAS Number | 6493-05-6 | Molecular Weight | 278.307 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 531.3±56.0 °C at 760 mmHg | |
| Molecular Formula | C13H18N4O3 | Melting Point | 98-100°C | |
| MSDS | Chinese USA | Flash Point | 275.1±31.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of PentoxifyllinePentoxifylline is a competitive nonselective phosphodiesterase inhibitor.Target: PDEPentoxifylline is a competitive nonselective phosphodiesterase inhibitor which raises intracellular cAMP, activates PKA, inhibits TNF and leukotriene synthesis, and reduces inflammation and innate immunity. In addition, pentoxifylline improves red blood cell deformability, reduces blood viscosity and decreases the potential for platelet aggregation and thrombus formation. Pentoxifylline is also an antagonist at adenosine 2 receptors [1]. Pentoxifylline is generally well tolerated. Based on the totality of the available evidence, it is possible that pentoxifylline could have a place in the treatment of IC as a means of improving walking distance and as a complimentary treatment assuming all other essential measures such as lifestyle change, exercise and treatment for secondary prevention have been taken into account [2]. Pentoxifylline reduce AST and ALT levels and may improve liver histological scores in patients with NALFD/NASH, but did not appear to affect cytokines. Large, prospective, and well-designed randomized, controlled studies are needed to address this issue [3]. |
| Name | Pentoxifylline |
|---|---|
| Synonym | More Synonyms |
| Description | Pentoxifylline is a competitive nonselective phosphodiesterase inhibitor.Target: PDEPentoxifylline is a competitive nonselective phosphodiesterase inhibitor which raises intracellular cAMP, activates PKA, inhibits TNF and leukotriene synthesis, and reduces inflammation and innate immunity. In addition, pentoxifylline improves red blood cell deformability, reduces blood viscosity and decreases the potential for platelet aggregation and thrombus formation. Pentoxifylline is also an antagonist at adenosine 2 receptors [1]. Pentoxifylline is generally well tolerated. Based on the totality of the available evidence, it is possible that pentoxifylline could have a place in the treatment of IC as a means of improving walking distance and as a complimentary treatment assuming all other essential measures such as lifestyle change, exercise and treatment for secondary prevention have been taken into account [2]. Pentoxifylline reduce AST and ALT levels and may improve liver histological scores in patients with NALFD/NASH, but did not appear to affect cytokines. Large, prospective, and well-designed randomized, controlled studies are needed to address this issue [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 531.3±56.0 °C at 760 mmHg |
| Melting Point | 98-100°C |
| Molecular Formula | C13H18N4O3 |
| Molecular Weight | 278.307 |
| Flash Point | 275.1±31.8 °C |
| Exact Mass | 278.137878 |
| PSA | 78.89000 |
| LogP | 0.32 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.621 |
| Storage condition | Store at RT |
| Water Solubility | H2O: ≥43 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | XH2475000 |
| HS Code | 2933990090 |
|
~% 
Pentoxifylline CAS#:6493-05-6 |
| Literature: Journal of the Chemical Society - Perkin Transactions 1, , # 6 p. 677 - 680 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Modulation of cytochrome P450 2A5 activity by lipopolysaccharide: low-dose effects and non-monotonic dose-response relationship.
PLoS ONE 10(1) , e0117842, (2015) Mouse cytochrome P450 (CYP) 2A5 is induced by inflammatory conditions and infectious diseases that down-regulate the expression and activity of most other CYP isoforms. Enhanced oxidative stress and n... |
|
|
Plasma matrix metalloproteinase activity in horses after intravenous infusion of lipopolysaccharide and treatment with matrix metalloproteinase inhibitors.
Am. J. Vet. Res. 74(3) , 473-80, (2013) To establish an in vivo method for matrix metalloproteinase (MMP)-2 and MMP-9 induction in horses via IV administration of lipopolysaccharide (LPS) and to evaluate the ability of doxycycline, oxytetra... |
|
|
Pentoxifylline for slow to resolve hepatopulmonary syndrome post liver transplantation: helpful or unnecessary?
Acta Gastroenterol. Belg. 76(1) , 70-1, (2013)
|
| Azupentat |
| 3,7-dimethyl-1-(5-oxohexyl)purine-2,6-dione |
| 3,7-Dimethyl-1-(5-oxohexyl)-3,7-dihydro-1H-purine-2,6-dione |
| 1H-Purine-2,6-dione, 3,7-dihydro-3,7-dimethyl-1-(5-oxohexyl)- |
| EINECS 229-374-5 |
| Durapental |
| Agapurin Retard |
| Trental |
| Pentoxifylline |
| PTX |
| Rentylin |
| Pentoxil |
| MFCD00063379 |
| Oxpentifylline |

 CAS#:6493-06-7
CAS#:6493-06-7
