7-Deazaadenosine(Tubercidin)
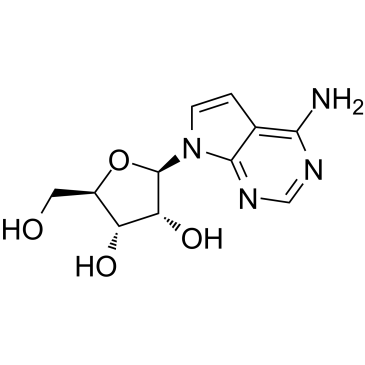
7-Deazaadenosine(Tubercidin) structure
|
Common Name | 7-Deazaadenosine(Tubercidin) | ||
|---|---|---|---|---|
| CAS Number | 69-33-0 | Molecular Weight | 266.253 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 648.8±55.0 °C at 760 mmHg | |
| Molecular Formula | C11H14N4O4 | Melting Point | 247-248 °C (decomp) | |
| MSDS | Chinese USA | Flash Point | 346.2±31.5 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of 7-Deazaadenosine(Tubercidin)Tubercidin (7-Deazaadenosine) is an adenosine analog, is an antibiotic obtained from Streptomyces tubercidicus.Target: AntibacterialTubercidin inhibits the growth of Streptococcus faecalis by 50 % at a concentration of 20 nM. Tubercidin is not subject to cleavage by adenosine phosphorylase or to deamination by adenosine deaminase. The antibiotic served as a substrate for numerous enzymes involved in the anabolism of adenosine, as demonstrated by its incorporation into RNA and DNA, and by the formation of nicotinamide-deaza-adenine dinucleotide. Tubercidin proves to be a weak inhibitor of adenosine phosphorylase, and interfered with the phosphorylation of adenosine and AMP. The inhibition of the growth of S. faecalis by Tubercidin is prevented by purine and pyrimidine nucleosides, ribose 5-phosphate, pyruvate, and certain amino acids. In the presence of Tubercidin, growing cultures of the test organism used pyruvate instead of glucose, whereas in the absence of the antibiotic glucose served as the main source of energy. It is suggested, therefore, that the impairment of growth is due primarily to the interference of Tubercidin with the utilization of glucose. |
| Name | tubercidin |
|---|---|
| Synonym | More Synonyms |
| Description | Tubercidin (7-Deazaadenosine) is an adenosine analog, is an antibiotic obtained from Streptomyces tubercidicus.Target: AntibacterialTubercidin inhibits the growth of Streptococcus faecalis by 50 % at a concentration of 20 nM. Tubercidin is not subject to cleavage by adenosine phosphorylase or to deamination by adenosine deaminase. The antibiotic served as a substrate for numerous enzymes involved in the anabolism of adenosine, as demonstrated by its incorporation into RNA and DNA, and by the formation of nicotinamide-deaza-adenine dinucleotide. Tubercidin proves to be a weak inhibitor of adenosine phosphorylase, and interfered with the phosphorylation of adenosine and AMP. The inhibition of the growth of S. faecalis by Tubercidin is prevented by purine and pyrimidine nucleosides, ribose 5-phosphate, pyruvate, and certain amino acids. In the presence of Tubercidin, growing cultures of the test organism used pyruvate instead of glucose, whereas in the absence of the antibiotic glucose served as the main source of energy. It is suggested, therefore, that the impairment of growth is due primarily to the interference of Tubercidin with the utilization of glucose. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 648.8±55.0 °C at 760 mmHg |
| Melting Point | 247-248 °C (decomp) |
| Molecular Formula | C11H14N4O4 |
| Molecular Weight | 266.253 |
| Flash Point | 346.2±31.5 °C |
| Exact Mass | 266.101501 |
| PSA | 126.65000 |
| LogP | -0.12 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.834 |
| InChIKey | HDZZVAMISRMYHH-KCGFPETGSA-N |
| SMILES | Nc1ncnc2c1ccn2C1OC(CO)C(O)C1O |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | P264-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T+: Very toxic; |
| Risk Phrases | R28 |
| Safety Phrases | S45;S36/S37/S39 |
| RIDADR | UN 3462 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | UY8870000 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 29419090 |
| Precursor 8 | |
|---|---|
| DownStream 6 | |
|
Plasmodium falciparum: the potential of the cancer chemotherapeutic agent cisplatin and its analogues as anti-malarials.
Exp. Parasitol. 132(4) , 440-3, (2012) The anti-malarial activity of the cancer chemotherapeutic agent cisplatin and cisplatin analogues was determined in Plasmodium falciparum. The cisplatin analogues included DNA-targeted acridine-tether... |
|
|
Sangivamycin-like molecule 6 exhibits potent anti-multiple myeloma activity through inhibition of cyclin-dependent kinase-9.
Mol. Cancer Ther. 11(11) , 2321-30, (2012) Despite significant treatment advances over the past decade, multiple myeloma (MM) remains largely incurable. In this study we found that MM cells were remarkably sensitive to the death-inducing effec... |
|
|
Insights into phosphate cooperativity and influence of substrate modifications on binding and catalysis of hexameric purine nucleoside phosphorylases.
PLoS ONE 7(9) , e44282, (2012) The hexameric purine nucleoside phosphorylase from Bacillus subtilis (BsPNP233) displays great potential to produce nucleoside analogues in industry and can be exploited in the development of new anti... |
| EINECS 200-703-4 |
| Tubercidine |
| sparsomycina |
| 7H-pyrrolo[2,3-d]pyrimidin-4-amine, 7-β-D-ribofuranosyl- |
| MFCD00056012 |
| TUBERCIDIN |
| 4-amino-7-(D-ribofuranosyl)-7H-pyrrolo{2,3-d}pyrimidine |
| 7-Deazaadenosine |
| Sparsomycine A |
 CAS#:16754-80-6
CAS#:16754-80-6![4-(methylsulfonyl)-7-(β-D-ribofuranosyl)-7H-pyrrolo[2,3-d]pyrimidine Structure](https://image.chemsrc.com/caspic/200/120401-34-5.png) CAS#:120401-34-5
CAS#:120401-34-5 CAS#:75921-20-9
CAS#:75921-20-9 CAS#:115479-39-5
CAS#:115479-39-5 CAS#:85572-94-7
CAS#:85572-94-7![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine Structure](https://image.chemsrc.com/caspic/189/3680-69-1.png) CAS#:3680-69-1
CAS#:3680-69-1![7-[5-O-[(1,1-dimethylethyl)dimethylsilyl]-2,3-O-(1-methylethylidene)-β-D-ribofuranosyl]-4-(methylthio)-7H-pyrrolo[2,3-d]pyrimidine Structure](https://image.chemsrc.com/caspic/217/120401-29-8.png) CAS#:120401-29-8
CAS#:120401-29-8![7H-Pyrrolo[2,3-d]pyrimidine,4-(methylthio)-7-b-D-ribofuranosyl- Structure](https://image.chemsrc.com/caspic/039/16754-86-2.png) CAS#:16754-86-2
CAS#:16754-86-2![7H-Pyrrolo[2,3-d]pyrimidine,4-methoxy-7-b-D-ribofuranosyl- structure](https://image.chemsrc.com/caspic/239/16754-81-7.png) CAS#:16754-81-7
CAS#:16754-81-7![1H-Pyrrolo[2,3-d]pyrimidin-4-amin structure](https://image.chemsrc.com/caspic/367/1500-85-2.png) CAS#:1500-85-2
CAS#:1500-85-2![1-(4-amino-pyrrolo[2,3-d]pyrimidin-7-yl)-β-D-1-deoxy-ribofuranose structure](https://image.chemsrc.com/caspic/188/72933-38-1.png) CAS#:72933-38-1
CAS#:72933-38-1![2-(5-dimethylamino-2,4,9-triazabicyclo[4.3.0]nona-2,4,7,10-tetraen-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol structure](https://image.chemsrc.com/caspic/489/20371-00-0.png) CAS#:20371-00-0
CAS#:20371-00-0![Methanimidamide,N,N-dimethyl-N'-(7-b-D-ribofuranosyl-7H-pyrrolo[2,3-d]pyrimidin-4-yl)- structure](https://image.chemsrc.com/caspic/328/57881-19-3.png) CAS#:57881-19-3
CAS#:57881-19-3 CAS#:40627-30-3
CAS#:40627-30-3
