Cephalomannine
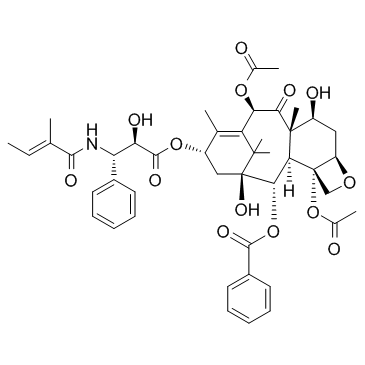
Cephalomannine structure
|
Common Name | Cephalomannine | ||
|---|---|---|---|---|
| CAS Number | 71610-00-9 | Molecular Weight | 831.901 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 929.5±65.0 °C at 760 mmHg | |
| Molecular Formula | C45H53NO14 | Melting Point | 139-141ºC | |
| MSDS | Chinese USA | Flash Point | 516.0±34.3 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
Use of CephalomannineCephalomannine is a taxol derivative with antitumor, antiproliferative properties. IC50 value:Target: Cephalomannine is an active anti-cancer agent obtained from Taxus yunnanensis and has an antineoplastic effect on tumors found in mice. Cephalomannine is a chemotherapy drug that is given as a treatment for some types of cancer. Cephalomannine is most commonly used to treat non-small cell lung cancer. |
| Name | Cephalomannine |
|---|---|
| Synonym | More Synonyms |
| Description | Cephalomannine is a taxol derivative with antitumor, antiproliferative properties. IC50 value:Target: Cephalomannine is an active anti-cancer agent obtained from Taxus yunnanensis and has an antineoplastic effect on tumors found in mice. Cephalomannine is a chemotherapy drug that is given as a treatment for some types of cancer. Cephalomannine is most commonly used to treat non-small cell lung cancer. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 929.5±65.0 °C at 760 mmHg |
| Melting Point | 139-141ºC |
| Molecular Formula | C45H53NO14 |
| Molecular Weight | 831.901 |
| Flash Point | 516.0±34.3 °C |
| Exact Mass | 831.346619 |
| PSA | 221.29000 |
| LogP | 6.59 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.615 |
| InChIKey | DBXFAPJCZABTDR-BDUDMYRDSA-N |
| SMILES | CC=C(C)C(=O)NC(c1ccccc1)C(O)C(=O)OC1CC2(O)C(OC(=O)c3ccccc3)C3C4(OC(C)=O)COC4CC(O)C3(C)C(=O)C(OC(C)=O)C(=C1C)C2(C)C |
| Storage condition | -20°C Freezer |
| Water Solubility | DMSO: 14 mg/mL, soluble |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H318-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi |
| Risk Phrases | 37/38-41 |
| Safety Phrases | 26-39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2932999022 |
|
~% 
Cephalomannine CAS#:71610-00-9 |
| Literature: US2008/287696 A1, ; Page/Page column 6 ; |
|
~% 
Cephalomannine CAS#:71610-00-9 |
| Literature: Roh, Eun Joo; Kim, Deukjoon; Lee, Chong Ock; Choi, Sang Un; Song, Choong Eui Bioorganic and medicinal chemistry, 2002 , vol. 10, # 10 p. 3145 - 3151 |
|
~% 
Cephalomannine CAS#:71610-00-9 |
| Literature: Bioorganic and medicinal chemistry, , vol. 10, # 10 p. 3145 - 3151 |
|
~% 
Cephalomannine CAS#:71610-00-9 |
| Literature: Bioorganic and medicinal chemistry, , vol. 10, # 10 p. 3145 - 3151 |
|
~% 
Cephalomannine CAS#:71610-00-9 |
| Literature: Bioorganic and medicinal chemistry, , vol. 10, # 10 p. 3145 - 3151 |
| Precursor 2 | |
|---|---|
| DownStream 8 | |
| HS Code | 2932999022 |
|---|
|
Determination of paclitaxel and related taxanes in bulk drug and injectable dosage forms by reversed phase liquid chromatography.
Anal. Chem. 69(11) , 2008-16, (1997) Baseline separation of 15 taxanes including paclitaxel (Taxol) was achieved on pentafluorophenyl (PFP) HPLC columns. Methods using aqueous acetonitrile gradients on each of two commercial PFP columns ... |
|
|
Determination of cephalomannine in rat plasma by gradient elution UPLC-MS/MS method.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 963 , 70-4, (2014) A rapid, sensitive and selective ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) was developed and validated for the determination and pharmacokinetic investigation of ce... |
|
|
Microbial transformation of cephalomannine by Luteibacter sp.
J. Nat. Prod. 70(12) , 1846-9, (2007) Luteibacter sp., a new bacterium isolated from the soil around a Taxus cuspidata Sieb. et Zucc plant, was studied for its capability to metabolize cephalomannine (1). After preparative fermentation, e... |
| Paclitaxel Related CoMpound A |
| (2α,5β,7β,10β,13α)-4,10-Diacetoxy-1,7-dihydroxy-13-{[(2R,3S)-2-hydroxy-3-{[(2E)-2-methylbut-2-enoyl]amino}-3-phenylpropanoyl]oxy}-9-oxo-5,20-epoxytax-11-en-2-yl benzoate |
| Benzenepropanoic acid, α-hydroxy-β-[[(2E)-2-methyl-1-oxo-2-buten-1-yl]amino]-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecah ydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)- |
| benzenepropanoic acid, α-hydroxy-β-[[(2E)-2-methyl-1-oxo-2-buten-1-yl]amino]-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)- |
| chephalomannine |
| (2α,5β,7β,10β,13α)-4,10-Diacetoxy-1,7-dihydroxy-13-{[(2R,3S)-2-hydroxy-3-{[(2E)-2-methyl-2-butenoyl]amino}-3-phenylpropanoyl]oxy}-9-oxo-5,20-epoxytax-11-en-2-yl benzoate |
| 3'-N-(trans-2-methyl-2-butenoyl)-3'-N-debenzoylpaclitaxel |
| CEPHALOMANNINE:BENZENEPROPANOICACID,-HYDROXY--[(2-METHYL-1-OXO-2-BUTENYL)AMINO]-,6,12B-BIS(ACETYLOXY)-12-(BENZOYLOXY)-2A,3,4,4A,5,6,9,10,11,12,12A,12B-DODECAHYDRO-4,11-DIHYDROXY-4A,8,13,13-TETRAMETHYL-5-OXO-7,11-METHANO-1H-CYCLODECA[3,4]BENZ[1,2-B]OX... |
| caphalomannine |
| Paclitaxel Related Compound A (20 mg) (Ce-phalomannine) |
| (2α,5β,7β,10β,13α)-4,10-bis(acetyloxy)-1,7-dihydroxy-13-{[(2R,3S)-2-hydroxy-3-{[(2E)-2-methylbut-2-enoyl]amino}-3-phenylpropanoyl]oxy}-9-oxo-5,20-epoxytax-11-en-2-yl benzoate |
| TAXOL B |
| CEPHALOMANNINE:BENZENEPROPANOICACID,-HYDROXY--[(2-METHYL-1-OXO-2-BUTENYL)AMINO]-,6,12B-BIS(ACETYLOXY)-12-(BENZOYLOXY)-2A,3,4,4A,5,6,9,10,11,12,12A,12B-DODECAHYDRO-4,11-DIHYDROXY-4A,8,13,13-TETRAMETHYL-5-OXO-7,11-METHANO-1H-CYCLODECA[3,4]BENZ[1 |
| CEPHALOMANNINE (TAXOL B) |
| CEPHALOMANNINE(P) |


 CAS#:32981-86-5
CAS#:32981-86-5 CAS#:71610-01-0
CAS#:71610-01-0 CAS#:27548-93-2
CAS#:27548-93-2 CAS#:76480-33-6
CAS#:76480-33-6 CAS#:76429-85-1
CAS#:76429-85-1 CAS#:71629-92-0
CAS#:71629-92-0 CAS#:33069-62-4
CAS#:33069-62-4 CAS#:133524-70-6
CAS#:133524-70-6
