(+/-)-Camphor
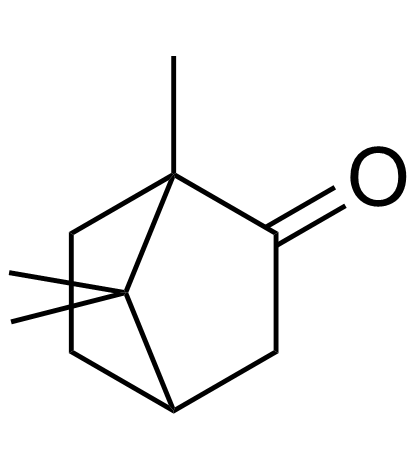
(+/-)-Camphor structure
|
Common Name | (+/-)-Camphor | ||
|---|---|---|---|---|
| CAS Number | 76-22-2 | Molecular Weight | 152.233 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 207.4±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H16O | Melting Point | 175-177 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 64.4±0.0 °C | |
| Symbol |



GHS02, GHS07, GHS08 |
Signal Word | Warning | |
Use of (+/-)-CamphorCamphor ((±)-Camphor) is a topical anti-infective and anti-pruritic and internally as a stimulant and carminative. However, Camphor is poisonous when ingested. Antiviral, antitussive, and anticancer activities[1]. Camphor is a TRPV3 agonist[2]. |
| Name | camphor |
|---|---|
| Synonym | More Synonyms |
| Description | Camphor ((±)-Camphor) is a topical anti-infective and anti-pruritic and internally as a stimulant and carminative. However, Camphor is poisonous when ingested. Antiviral, antitussive, and anticancer activities[1]. Camphor is a TRPV3 agonist[2]. |
|---|---|
| Related Catalog | |
| Target |
TRPV3[2] |
| In Vitro | Camphor induces fibroblast proliferation through the PI3K/AKT and ERK signaling pathways. The MTT assay results show that 32.5, 65, 130, and 260 μM Camphor increase fibroblast viability to 108.9±6.6%, 118.6±2.8%, 127.7±4.2%, and 131.6±7.2%, respectively, compared to 0 μM Camphor treatment. Camphor treatment for 24 h increases the generation of ROS by up to 17.97% compared to 5.04% in the no-treatment control[3]. |
| Cell Assay | Primary dermal fibroblast (2×104 cells/mL) cells are plated in 96-well plates in 200μL of medium containing 10% FBS. After 24 h, the cells are treated with various concentrations of chamomile hydrosol or Camphor (0-260 μM). After treatment, 20 μL of MTT solution (5mg/mL) is added to each well, followed by incubation in a humidified environment for 3-4 h. The supernatant is removed and 150 μL of DMSO is added. Cell viability is determined from the absorbance at 570 nm, measured using a Sunrise microplate reader[3]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 207.4±0.0 °C at 760 mmHg |
| Melting Point | 175-177 °C(lit.) |
| Molecular Formula | C10H16O |
| Molecular Weight | 152.233 |
| Flash Point | 64.4±0.0 °C |
| Exact Mass | 152.120117 |
| PSA | 17.07000 |
| LogP | 2.13 |
| Vapour density | 5.2 (vs air) |
| Vapour Pressure | 0.2±0.4 mmHg at 25°C |
| Index of Refraction | 1.485 |
| InChIKey | DSSYKIVIOFKYAU-UHFFFAOYSA-N |
| SMILES | CC12CCC(CC1=O)C2(C)C |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, metallic salts, combustible materials, organics. |
| Water Solubility | 0.12 g/100 mL (25 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H228-H302 + H332-H371 |
| Precautionary Statements | P210-P260-P301 + P312 + P330-P370 + P378 |
| Target Organs | Lungs |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | F:Flammable |
| Risk Phrases | R11;R36/37/38 |
| Safety Phrases | S16-S26-S37/39 |
| RIDADR | UN 2717 4.1/PG 3 |
| WGK Germany | 1 |
| RTECS | EX1225000 |
| Packaging Group | III |
| Hazard Class | 4.1 |
| HS Code | 2914291000 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2914291000 |
|---|
|
Carvacrol and rosemary essential oil manifest cytotoxic, DNA-protective and pro-apoptotic effect having no effect on DNA repair.
Neoplasma 61(6) , 690-9, (2014) For several thousand years natural products were successfully used to treat a variety of diseases and to maintain health in humans, but until now it is not fully known what causes these medicinal effe... |
|
|
In vitro antifungal activity and probable fungicidal mechanism of aqueous extract of Barleria grandiflora.
Appl. Biochem. Biotechnol. 175(8) , 3571-84, (2015) Barleria grandiflora Dalz. (Acanthaceae) is being used in India to treat different types of disorders including skin infections. Therefore, there are good possibilities to find antifungal compounds in... |
|
|
Development and application of a non-targeted extraction method for the analysis of migrating compounds from plastic baby bottles by GC-MS.
Food Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk Assess. 31(12) , 2090-102, (2014) In 2011, the European Union prohibited the production of polycarbonate (PC) baby bottles due to the toxic effects of the PC monomer bisphenol-A. Therefore, baby bottles made of alternative materials, ... |
| (+)-bornan-2-one |
| Bicyclo(2.2.1)heptan-2-one, 1,7,7-trimethyl- |
| camphanone |
| 3-Fluor-2,2-dimethyl-bicyclo<2.2.1>heptan |
| (1S,4S)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one |
| 1,7,7-Trimethylbicyclo[2.2.1]-2-heptanone |
| 2-Kamfanon |
| D-CAMPHOR |
| (±)-bornan-2-one |
| 2-Oxo-bornan |
| 2-Bornanone,2-Camphanone,D-Camphor |
| Radian B |
| lphanon |
| DL-2-Bornanone |
| D-(+)-Camphor |
| MFCD00676613 |
| Alphanon |
| L(-)-Camphor |
| 1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one |
| DL-Camphor |
| Camphen-hydrofluorid |
| (1RS,4RS)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one |
| Caladryl |
| (-)-Camphor |
| 2-Keto-1,7,7-trimethylnolcamphane |
| Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl- |
| (−)-Camphor,(1S)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one |
| 2-CAMPHOR |
| EINECS 200-945-0 |
| (±)-Camphor |
| 2-Camphonone |
| Dehydrocamphor |
| Camphor |
| (+)-CaMphor |
| (1R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one |
| 2-Bornanone,2-Camphanone |
| D(+)-Camphor |
| 2-Bornanone 2-Camphanone |
| Kampfer |
| (+)-Camphor,(1R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one |
| camphre |
| (1S)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one |
 CAS#:24393-70-2
CAS#:24393-70-2 CAS#:6627-72-1
CAS#:6627-72-1 CAS#:18529-99-2
CAS#:18529-99-2 CAS#:124-76-5
CAS#:124-76-5![()-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one oxime Structure](https://image.chemsrc.com/caspic/300/18674-50-5.png) CAS#:18674-50-5
CAS#:18674-50-5![4',7',7'-trimethylspiro[1,3-dithiolane-2,3'-bicyclo[2.2.1]heptane] Structure](https://image.chemsrc.com/caspic/379/6787-91-3.png) CAS#:6787-91-3
CAS#:6787-91-3 CAS#:76-29-9
CAS#:76-29-9 CAS#:507-70-0
CAS#:507-70-0 CAS#:7519-74-6
CAS#:7519-74-6 CAS#:32511-35-6
CAS#:32511-35-6 CAS#:32511-34-5
CAS#:32511-34-5 CAS#:527-53-7
CAS#:527-53-7 CAS#:4501-58-0
CAS#:4501-58-0 CAS#:464-45-9
CAS#:464-45-9 CAS#:464-43-7
CAS#:464-43-7
