Campathecin
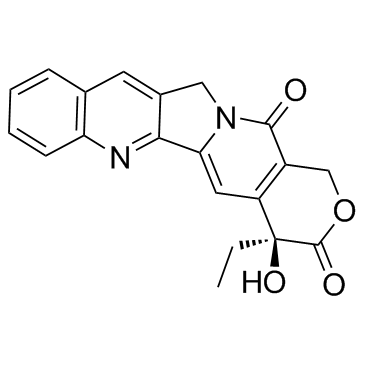
Campathecin structure
|
Common Name | Campathecin | ||
|---|---|---|---|---|
| CAS Number | 7689-03-4 | Molecular Weight | 348.352 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 757.0±60.0 °C at 760 mmHg | |
| Molecular Formula | C20H16N2O4 | Melting Point | 260 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 411.6±32.9 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of CampathecinCampathecin is a potent DNA enzyme topoisomerase I inhibitor, with an IC50 of 679 nM. |
| Name | camptothecin |
|---|---|
| Synonym | More Synonyms |
| Description | Campathecin is a potent DNA enzyme topoisomerase I inhibitor, with an IC50 of 679 nM. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I:679 nM (IC50) |
| In Vitro | [3H]BrCPT labeling of topoisomerase I is enhanced greatly by the presence of DNA; very little labeling of isolated topoisomerase I or isolated DNA occurrs. Even in the presence of DNA, [3H]BrCPT labeling of topoisomerase I is inhibited by camptothecin, suggesting that both CPT and BrCPT bind to the same site on the DNA-topoisomerase I binary complex[1]. With increasing concentrations of camptothecin, closed circular pRR322 DNA (form I) is converted to nicked circular DNA (form 11). This apparent nicking activitv of camptothecin required DNA topoisomerase I[2]. |
| Kinase Assay | Each reaction mixture (200 μL, total volume) contained 2 μg of supercoiled pDPT2789 DNA (2.4 nM plasmid concentration), 50 mM Tris-HCl, pH 7.5, 120 mM KCl, 10 mM MgCl2, 0.5 mM EDTA, 0.05% dimethyl sulfoxide, 1% methanol, 100 μg/mL BSA, 4.3 ng of calf thymus topoisomerase I (0.26 nM), and a CPT derivative. The reaction mixture is incubated at 37°C, and at the indicated times 20-μL aliquots are removed and terminated by the addition of 5 μL of SDS/Ficoll stop mix (final concentrations, 0.5% SDS, 2% Ficoll, 0.025% bromphenol blue). The samples are loaded onto a 1% agarose gel and analyzed by electrophoresis. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 757.0±60.0 °C at 760 mmHg |
| Melting Point | 260 °C (dec.)(lit.) |
| Molecular Formula | C20H16N2O4 |
| Molecular Weight | 348.352 |
| Flash Point | 411.6±32.9 °C |
| Exact Mass | 348.110992 |
| PSA | 81.42000 |
| LogP | 1.60 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.746 |
| InChIKey | VSJKWCGYPAHWDS-FQEVSTJZSA-N |
| SMILES | CCC1(O)C(=O)OCc2c1cc1n(c2=O)Cc2cc3ccccc3nc2-1 |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S45-S36/37/39-S26 |
| RIDADR | UN 1544 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | UQ0492000 |
| Precursor 6 | |
|---|---|
| DownStream 9 | |
|
TAp73 promotes cell survival upon genotoxic stress by inhibiting p53 activity.
Oncotarget 5(18) , 8107-22, (2014) p53 plays a key role in regulating DNA damage response by suppressing cell cycle progression or inducing apoptosis depending on extent of DNA damage. However, it is not clear why mild genotoxic stress... |
|
|
DNA damage-specific deubiquitination regulates Rad18 functions to suppress mutagenesis.
J. Cell Biol. 206(2) , 183-97, (2014) Deoxyribonucleic acid (DNA) lesions encountered during replication are often bypassed using DNA damage tolerance (DDT) pathways to avoid prolonged fork stalling and allow for completion of DNA replica... |
|
|
A dihydroselenoquinazoline inhibits S6 ribosomal protein signalling, induces apoptosis and inhibits autophagy in MCF-7 cells.
Eur. J. Pharm. Sci. 63 , 87-95, (2014) The PI3K/Akt/mTOR/S6 ribosomal protein signalling pathway is a key potential target in breast cancer therapy, playing a central role in proliferation and cell survival. In this study, we found that th... |
| EINECS 444-280-6 |
| Camptothecin |
| d-Camptothecin |
| 1H-Pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-, (S)- |
| (S)-4-ethyl-4-hydroxy-1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione |
| (S)-4-ethyl-4-hydroxy-1H-Pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione |
| CaMpathecin |
| 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-, (4S)- |
| (S)-(+)-Camptothecin |
| (4S)-4-Ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione |
| 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-, (S)- |
| Camptohecin |
| MFCD00081076 |
| Baicalin std. |
| CAMPTOTHECINE |
| 1H-Pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-, (4S)- |
| (S)-4-ethyl-4-hydroxy-1H-pyrano-[3',4':6,7]indolizinol[1,2-b]quinoline-3,14(4H,12H)-dione |
| (+)-Camptothecin |
| (+)-Camptothecine |
![4-ethyl-1,12-dihydro-14H-pyrano[3',4':6,7indolizino[1,2-b]]quinolin-14-one Structure](https://image.chemsrc.com/caspic/481/841276-70-8.png) CAS#:841276-70-8
CAS#:841276-70-8![(S)-4-Ethyl-4-hydroxy-6-iodo-3-oxo-7-propargyl-1H-pyrano[3,4-c]-8-pyridone Structure](https://image.chemsrc.com/caspic/174/174092-80-9.png) CAS#:174092-80-9
CAS#:174092-80-9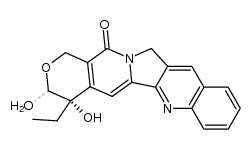 CAS#:210563-62-5
CAS#:210563-62-5![2-[(4-methylphenyl)iminomethyl]aniline Structure](https://image.chemsrc.com/caspic/359/55857-35-7.png) CAS#:55857-35-7
CAS#:55857-35-7![(±)- 1H-Pyrano[3,4-f]indolizine-3,6,10(4H)-trione, 4-ethyl-7,8-dihydro-4-hydroxy Structure](https://image.chemsrc.com/caspic/342/102978-40-5.png) CAS#:102978-40-5
CAS#:102978-40-5![(S)-4-ethyl-4-hydroxy-7,8-dihydro-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione Structure](https://image.chemsrc.com/caspic/225/110351-94-5.png) CAS#:110351-94-5
CAS#:110351-94-5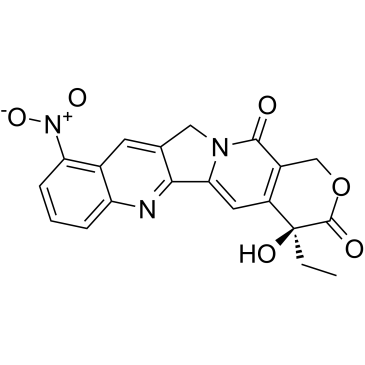 CAS#:91421-42-0
CAS#:91421-42-0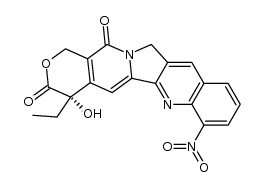 CAS#:58546-27-3
CAS#:58546-27-3 CAS#:91421-43-1
CAS#:91421-43-1![5-ethyl-5-hydroxy-1,4,5,13-tetrahydro-3H,15H-oxepino[3',4':6,7]indolizino[1,2-b]quinoline-3,15-dione structure](https://image.chemsrc.com/caspic/153/186668-40-6.png) CAS#:186668-40-6
CAS#:186668-40-6![1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione,4-ethyl-4-hydroxy- structure](https://image.chemsrc.com/caspic/436/31456-25-4.png) CAS#:31456-25-4
CAS#:31456-25-4 CAS#:86639-52-3
CAS#:86639-52-3 CAS#:97682-44-5
CAS#:97682-44-5 CAS#:19685-09-7
CAS#:19685-09-7![(4S)-4-Ethyl-4-hydroxy-9-methoxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione structure](https://image.chemsrc.com/caspic/000/19685-10-0.png) CAS#:19685-10-0
CAS#:19685-10-0
