rubitecan
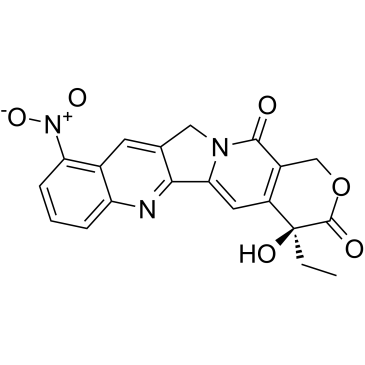
rubitecan structure
|
Common Name | rubitecan | ||
|---|---|---|---|---|
| CAS Number | 91421-42-0 | Molecular Weight | 393.350 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 816.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C20H15N3O6 | Melting Point | 182-186ºC | |
| MSDS | USA | Flash Point | 447.5±34.3 °C | |
Use of rubitecanRubitecan (RFS 2000), a camptothecin derivative, is an orally active topoisomerase I inhibitor with broad antitumor activity, and induces protein-linked DNA single-strand breaks, thereby blocking DNA and RNA synthesis in dividing cells[1][2][3]. |
| Name | 9-Nitrocamptothecin |
|---|---|
| Synonym | More Synonyms |
| Description | Rubitecan (RFS 2000), a camptothecin derivative, is an orally active topoisomerase I inhibitor with broad antitumor activity, and induces protein-linked DNA single-strand breaks, thereby blocking DNA and RNA synthesis in dividing cells[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I[1] |
| In Vitro | Rubitecan (RFS 2000) inhibits U-CH1, U-CH2, and CCL4 cells with IC50s 0.32, 0.83, and 7.7 µM, respectively[4]. |
| References |
[3]. Rubitecan |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 816.3±65.0 °C at 760 mmHg |
| Melting Point | 182-186ºC |
| Molecular Formula | C20H15N3O6 |
| Molecular Weight | 393.350 |
| Flash Point | 447.5±34.3 °C |
| Exact Mass | 393.096100 |
| PSA | 127.24000 |
| LogP | 1.38 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.762 |
| InChIKey | VHXNKPBCCMUMSW-FQEVSTJZSA-N |
| SMILES | CCC1(O)C(=O)OCc2c1cc1n(c2=O)Cc2cc3c([N+](=O)[O-])cccc3nc2-1 |
| Storage condition | Room temp |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | UQ0493300 |
| Precursor 6 | |
|---|---|
| DownStream 4 | |
|
Effect of injection routes on pharmacokinetics and lactone/carboxylate equilibrium of 9-Nitrocamptothecin in rats.
Int. J. Pharm. 340(1-2) , 29-33, (2007) Pharmacokinetics and lactone/carboxylate equilibrium of 9-Nitrocamptothecin (9-NC) were compared after intravenous (i.v.) and intramuscular (i.m.) injection at a dose of 1.5mg/kg 9-NC solution. The co... |
|
|
Effect of phospholipid composition on characterization of liposomes containing 9-nitrocamptothecin.
Drug Dev. Ind. Pharm. 32(6) , 719-26, (2006) 9-Nitrocamptothecin (9-NC), a newly developed camptothecin derivative, had poor solubility in any pharmaceutically acceptable solvents. One way of improving the solubility is to formulate the drug int... |
|
|
Sequential oral 9-nitrocamptothecin and etoposide: a pharmacodynamic- and pharmacokinetic-based phase I trial.
Mol. Cancer Ther. 5(8) , 2130-7, (2006) Resistance to topoisomerase (topo) I inhibitors has been related to down-regulation of nuclear target enzyme, whereas sensitization to topo II inhibitors may result from induction of topo II by topo I... |
| rubitecan |
| MFCD06656294 |
| 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-10-nitro-, (4S)- |
| PYRIDOXINE 3,4-DIPALMITATE |
| 9-nitro-20(s)-camptothecin |
| 1H-PYRANO[3',4':6,7]INDOLIZINO[1,2-B]QUINOLINE-3,14(4H,12H)-DIONE,4-ETHYL-4-HYDROXY-10-NITRO-, (4S)- |
| (4S)-4-Ethyl-4-hydroxy-10-nitro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione |
| Orathecin |
![(S)-4-ethyl-4-hydroxy-10-nitro-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl 4-nitrobenzenesulfonate Structure](https://image.chemsrc.com/caspic/085/172917-96-3.png) CAS#:172917-96-3
CAS#:172917-96-3![(S)-4-ethyl-4-hydroxy-10-nitro-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl benzenesulfonate Structure](https://image.chemsrc.com/caspic/161/172917-94-1.png) CAS#:172917-94-1
CAS#:172917-94-1![(S)-4-ethyl-4-hydroxy-10-nitro-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl 4-fluorobenzenesulfonate Structure](https://image.chemsrc.com/caspic/391/164159-91-5.png) CAS#:164159-91-5
CAS#:164159-91-5 CAS#:7689-03-4
CAS#:7689-03-4 CAS#:19685-09-7
CAS#:19685-09-7 CAS#:104267-73-4
CAS#:104267-73-4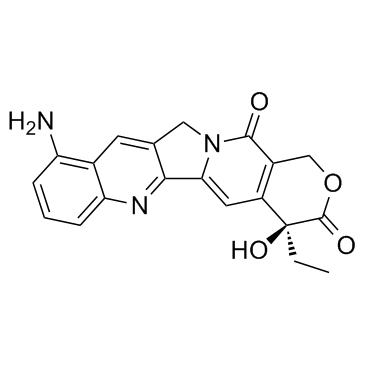 CAS#:91421-43-1
CAS#:91421-43-1 CAS#:67656-30-8
CAS#:67656-30-8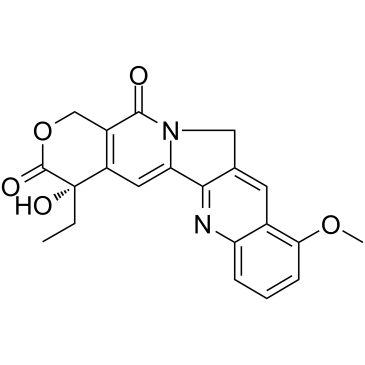 CAS#:39026-92-1
CAS#:39026-92-1
