9-Aminocamptothecin
Modify Date: 2025-08-20 19:49:08
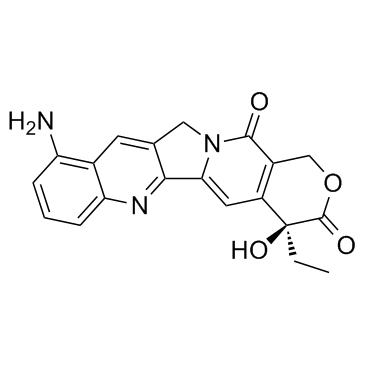
9-Aminocamptothecin structure
|
Common Name | 9-Aminocamptothecin | ||
|---|---|---|---|---|
| CAS Number | 91421-43-1 | Molecular Weight | 363.367 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 819.6±65.0 °C at 760 mmHg | |
| Molecular Formula | C20H17N3O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 449.5±34.3 °C | |
Use of 9-Aminocamptothecin9-Aminocamptothecin is a topoisomerase I inhibitor with potent anticancer activity. |
| Name | 9-Aminocamptothecin |
|---|---|
| Synonym | More Synonyms |
| Description | 9-Aminocamptothecin is a topoisomerase I inhibitor with potent anticancer activity. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I |
| In Vitro | In human breast (MCF-7), bladder (MGH-U1), and colon (HT-29) cancer cell lines, 9-Aminocamptothecin cytotoxicity increases with both higher drug concentrations and longer exposure times. Minimal cell killing is also observed unless 9-Aminocamptothecin concentrations exceeds a threshold of 2.7 nm[1]. 9-Aminocamptothecin inhibits PC-3, PC-3M, DU145, and LNCaP cells with IC50 values of 34.1, 10, 6.5, and 8.9 nM, respectively after 96 h of drug exposure[2]. |
| In Vivo | 9-Aminocamptothecin inhibits tumor growth at the lowest oral dose (0.35 mg/kg/day), whereas higher oral doses (0.75 and 1 mg/kg/day) and s.c. administration (4 mg/kg/week) causes tumor regression. 9-Aminocamptothecin is well tolerated at all doses, with no toxic death or weight loss of more than 10% observed in any group[2]. 9-Aminocamptothecin induces complete remissions in 55 % of SCID mice engrafted with human myeloid leukemia. The oral and intravenous routes are equally effective. The results with this pre-clinical model support the evaluation of 9-Aminocamptothecin as antileukemic agent in a phase I trial in patients with AML[3]. |
| Cell Assay | The cytotoxicity of 9-Aminocamptothecin is assessed by clonogenic assay. Exponentially growing cells are resuspended in media, cell number is determined using an electronic counter, and 100–250 cells are inoculated in triplicate onto 60 15-mm dishes containing 5 mL of medium. After an overnight incubation, 5 μL of 9-Aminocamptothecin stock solutions are added to the dishes to achieve final concentrations of 0, 0.27, 1.37, 2.74, 13.7, 27.4, 137, and 274 nM. After 4-, 8-, 12-, 24-, 48-, 72-, and 240-h exposures, medium is removed by aspiration and fresh medium is added to the dishes. Percentage of survival at each drug concentration with different exposure time is determined from the ratio of the number of the colonies in the drug-treated sample:the number in the control (DMSO vehicle-treated) sample[1]. |
| Animal Admin | Mice: Treatment with 9-Aminocamptothecin is started on day 7 after inoculation with KBM-3 cells. Five groups of 5 mice each (average weight of 22 g) are used and treatment is administered daily 4 days a week for 3 weeks as follows: 1) group 1 control mice are injected IV with PBS; 2) group 2 mice receive 1.33 mg/kg 9-Aminocamptothecin IV, 3) group 3 mice receive 1.33 mg/kg 9-Aminocamptothecin orally by gavage; 4) group 4 mice receive 2.0 mg/kg 9-Aminocamptothecin IV; 5) group 5 mice receive 2.0 mg/kg 9-Aminocamptothecin orally by gavage[3]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 819.6±65.0 °C at 760 mmHg |
| Molecular Formula | C20H17N3O4 |
| Molecular Weight | 363.367 |
| Flash Point | 449.5±34.3 °C |
| Exact Mass | 363.121918 |
| PSA | 107.44000 |
| LogP | 0.44 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.771 |
| InChIKey | FUXVKZWTXQUGMW-FQEVSTJZSA-N |
| SMILES | CCC1(O)C(=O)OCc2c1cc1n(c2=O)Cc2cc3c(N)cccc3nc2-1 |
| Storage condition | 2-8°C |
| Hazard Codes | T+ |
|---|---|
| RIDADR | 2811.0 |
| Hazard Class | 6.1 |
| HS Code | 2934999090 |
| Precursor 8 | |
|---|---|
| DownStream 3 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| (4S)-10-Amino-4-ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione |
| MFCD00909855 |
| Camptothecin,9-amino |
| 9-Amino-20-camptothecin |
| 9-Amino-camptothecin |
| 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 10-amino-4-ethyl-4-hydroxy-, (4S)- |
| 9-AC |
| 9-AMINO-20(S)-CAMPTOTHECIN |
| (20S)-9-aminocamptothecin |
| (S)-10-amino-4-ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione |
 CAS#:91421-42-0
CAS#:91421-42-0 CAS#:164159-99-3
CAS#:164159-99-3![(S)-4-ethyl-4-hydroxy-10-nitro-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl benzenesulfonate Structure](https://image.chemsrc.com/caspic/161/172917-94-1.png) CAS#:172917-94-1
CAS#:172917-94-1 CAS#:7689-03-4
CAS#:7689-03-4 CAS#:19685-09-7
CAS#:19685-09-7 CAS#:104267-73-4
CAS#:104267-73-4 CAS#:606-31-5
CAS#:606-31-5 CAS#:151585-78-3
CAS#:151585-78-3 CAS#:67656-30-8
CAS#:67656-30-8 CAS#:39026-92-1
CAS#:39026-92-1
