Pheniramine
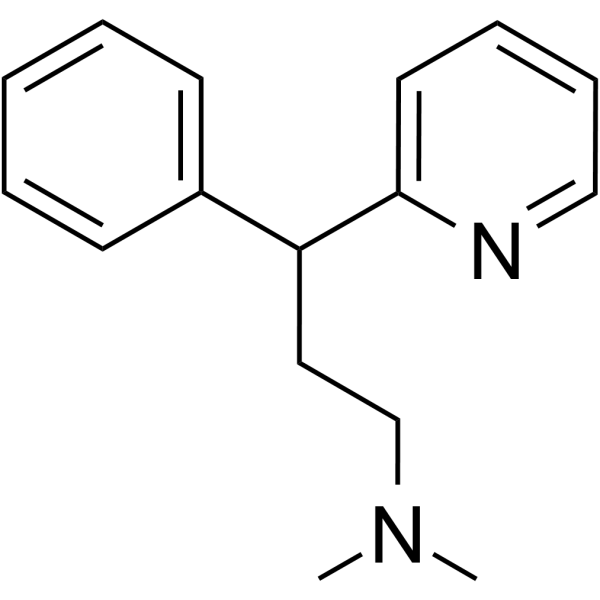
Pheniramine structure
|
Common Name | Pheniramine | ||
|---|---|---|---|---|
| CAS Number | 86-21-5 | Molecular Weight | 240.34300 | |
| Density | 1.018 g/cm3 | Boiling Point | 84 °C20 mm Hg(lit.) | |
| Molecular Formula | C16H20N2 | Melting Point | 30-34 °C(lit.) | |
| MSDS | N/A | Flash Point | 179 °F | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of PheniraminePheniramine (Prophenpyridamine;Tripoton) is a first-generation histamine H1 receptor antagonist, acts on the central nervous system (CNS) with sedative and hypnotic effect. Pheniramine displays antitumor effect and induces leukemia cells apoptosis. Pheniramine is also a safe and effective local anesthetic, with antipruritic effects[1][2][3][4]. |
| Name | N,N-dimethyl-3-phenyl-3-pyridin-2-ylpropan-1-amine |
|---|---|
| Synonym | More Synonyms |
| Description | Pheniramine (Prophenpyridamine;Tripoton) is a first-generation histamine H1 receptor antagonist, acts on the central nervous system (CNS) with sedative and hypnotic effect. Pheniramine displays antitumor effect and induces leukemia cells apoptosis. Pheniramine is also a safe and effective local anesthetic, with antipruritic effects[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
H1 Receptor:1.01 mM (IC50) |
| In Vitro | Pheniramine inhibits CYP2D6, the specific P450-isozymes, to delay metabolic time and prolong antihistaminic effects[1]. Pheniramine inhibits Ca2+ influx into BC3H-1 cells by inhibiting histamine with an IC50 value of 1.01 mM and regulates cellular Ca2+ transmembrane action[2]. Pheniramine (0.5, 1.0 mM; 24 h) induces cell apoptosis in human T-cell acute lymphoblastic leukemia cell lines[3]. Pheniramine (1 μM-1 mM; 12-48 h) inhibits cell proliferation in a time-dependent manner and shows inhibitory concentration IC50s of 550 μM (CCRF-CEM cells) and 420 μM (Jurkat cells), respectively[3]. Apoptosis Analysis[3] Cell Line: Human T-cell acute lymphoblastic leukemia cell lines: CCRF-CEM and Jurkat ALL Concentration: 0.5, 1.0 mM Incubation Time: 24 hours Result: Induced cells apoptosis with chromatin condenses and marginalizes, and nuclear debris spreaded into the cytoplasm. Cell Viability Assay[3] Cell Line: Human T-cell acute lymphoblastic leukemia cell lines: CCRF-CEM and Jurkat ALL Concentration: 1 μM-1 mM Incubation Time: 12, 24, 48 hours Result: Inhibited cell proliferation and survival in a time- and dose-dependent manner. |
| In Vivo | Pheniramine (1.75 μM; i.t.) exerts local anesthesia effect and results spinal block in rats[4]. Animal Model: Sprague–Dawley rats (300-350 g; male)[4] Dosage: 0.30, 0.60, 0.90, 1.50, 1.75 μM Administration: Intrathecal injection; one time Result: Resulted the spinal block and displayed dose-dependent effect. Showed 100% blockades in motor function, proprioception, and nociception, with full recoveryduration of action about 41, 56, and 88 min, respectively, at 1.75 μM. |
| References |
| Density | 1.018 g/cm3 |
|---|---|
| Boiling Point | 84 °C20 mm Hg(lit.) |
| Melting Point | 30-34 °C(lit.) |
| Molecular Formula | C16H20N2 |
| Molecular Weight | 240.34300 |
| Flash Point | 179 °F |
| Exact Mass | 240.16300 |
| PSA | 16.13000 |
| LogP | 3.16520 |
| Index of Refraction | 1.556 |
| Storage condition | ?20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | 1759 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2933399090 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Assessment of protective effects of methylprednisolone and pheniramine maleate on reperfusion injury in kidney after distant organ ischemia: a rat model.
Ann. Vasc. Surg. 26(4) , 559-65, (2012) Ischemia/reperfusion (I/R) injury of tissues is a common problem that cardiovascular surgeons are faced with. Suppression of inflammation, which plays an important role in the pathogenesis of I/R inju... |
|
|
Artificial neural network combined with principal component analysis for resolution of complex pharmaceutical formulations.
Chem. Pharm. Bull. 59(1) , 35-40, (2011) A chemometric approach based on the combined use of the principal component analysis (PCA) and artificial neural network (ANN) was developed for the multicomponent determination of caffeine (CAF), mep... |
|
|
C7a, a biphosphinic cyclopalladated compound, efficiently controls the development of a patient-derived xenograft model of adult T cell leukemia/lymphoma.
Viruses 3(7) , 1041-58, (2011) Adult T-cell leukemia/lymphoma (ATLL) is a highly aggressive disease that occurs in individuals infected with the human T lymphotropic virus type 1 (HTLV-1). Patients with aggressive ATLL have a poor ... |
| Histapyridamine |
| Avil |
| Propheniramine |
| Feniramine |
| pheniramine |
| N,N-dimethyl-3-phenyl-3-pyridin-2-yl-propan-1-amine |
| EINECS 201-656-2 |
| Trimeton |
| Prophenpyridamine |
| Pyriton |
| Tripoton |
| Metron |
| MFCD00865659 |
 CAS#:124-40-3
CAS#:124-40-3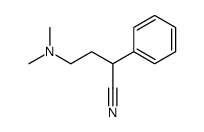 CAS#:50599-78-5
CAS#:50599-78-5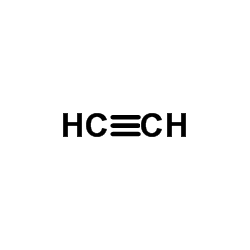 CAS#:74-86-2
CAS#:74-86-2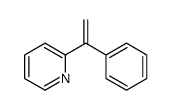 CAS#:15260-65-8
CAS#:15260-65-8 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:35344-68-4
CAS#:35344-68-4 CAS#:42817-44-7
CAS#:42817-44-7