Isoliquiritigenin
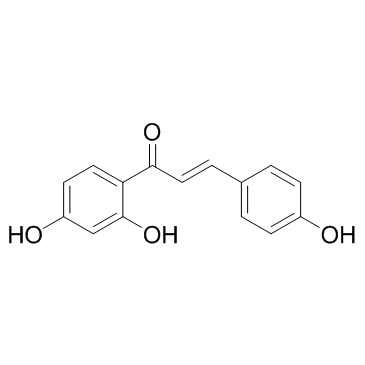
Isoliquiritigenin structure
|
Common Name | Isoliquiritigenin | ||
|---|---|---|---|---|
| CAS Number | 961-29-5 | Molecular Weight | 256.253 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 504.0±42.0 °C at 760 mmHg | |
| Molecular Formula | C15H12O4 | Melting Point | 206-210°C | |
| MSDS | Chinese USA | Flash Point | 272.7±24.4 °C | |
Use of IsoliquiritigeninIsoliquiritigenin is an anti-tumor flavonoid from the root of Glycyrrhiza glabra, which inhibits aldose reductase with an IC50 of 320 nM. |
| Name | isoliquiritigenin |
|---|---|
| Synonym | More Synonyms |
| Description | Isoliquiritigenin is an anti-tumor flavonoid from the root of Glycyrrhiza glabra, which inhibits aldose reductase with an IC50 of 320 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 320 nM (Aldose reductase) |
| In Vitro | Isoliquiritigenin may prevent diabetic complications through inhibiting rat lens aldose reductase with an IC50 of 320 nM and sorbitol accumulation in human red blood cells with an IC50 of 2.0 μM[1]. Isoliquiritigenin (100 μM) markedly inhibits the hypoxia-induced prolonged TPS and TR90 of cardiomyocytes. Isoliquiritigenin significantly triggers AMPK Thr172 phosphorylation as compared with vehicle group. Isoliquiritigenin treatment also induces extracellular signal-regulated kinase (ERK) signaling pathway in the cardiomyocytes. Isoliquiritigenin treatment significantly decreases the intracellular ROS levels of isolated cardiomyocytes during hypoxia/reoxygenation[3]. Isoliquiritigenin not only downregulates IL-6 expression but also significantly decreases levels of phosphorylated ERK and STAT3 and can inhibit phosphorylation levels of ERK and STAT3 induced by recombinant human IL-6, which are critical signaling proteins in IL-6 signaling regulation networks[4]. |
| In Vivo | Isoliquiritigenin shows concentration-dependent inhibition of the tonic contraction of mouse jejunum induced by various types of stimulants such as CCh (1 mM), KCl (60 mM) and BaCl2 (0.3 mM) with IC50 values of 4.96±1.97 mM, 4.03±1.34 mM and 3.70±0.58 mM, respectively[2]. Isoliquiritigenin exhibits significant anti-tumor activity in MM xenograft models and synergistically enhances the anti-myeloma activity of adriamycin[4]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 504.0±42.0 °C at 760 mmHg |
| Melting Point | 206-210°C |
| Molecular Formula | C15H12O4 |
| Molecular Weight | 256.253 |
| Flash Point | 272.7±24.4 °C |
| Exact Mass | 256.073547 |
| PSA | 77.76000 |
| LogP | 3.11 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.715 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: <0.1 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | FL7080000 |
| HS Code | 2914501900 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914501900 |
|---|---|
| Summary | 2914501900 other ketone-phenols。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Isoliquiritigenin attenuates oxidative hepatic damage induced by carbon tetrachloride with or without buthionine sulfoximine.
Chem. Biol. Interact. 225 , 13-20, (2015) Glycyrrhizae radix (G. radix) has been demonstrated to have hepatoprotective properties. This study determined the therapeutic effects of isoliquiritigenin (isoLQ) in G. radix, against liver injury in... |
|
|
Effects of phytochemicals on in vitro anti-inflammatory activity of Bifidobacterium adolescentis.
Biosci. Biotechnol. Biochem. 79 , 799-807, (2015) Probiotics have been shown to improve the condition of not only the human gastrointestinal tract but also the entire body. We found that quercetin enhances the anti-inflammatory activity of Bifidobact... |
|
|
Flavonoids and Auxin Transport Inhibitors Rescue Symbiotic Nodulation in the Medicago truncatula Cytokinin Perception Mutant cre1.
Plant Cell 27 , 2210-26, (2015) Initiation of symbiotic nodules in legumes requires cytokinin signaling, but its mechanism of action is largely unknown. Here, we tested whether the failure to initiate nodules in the Medicago truncat... |
| iso-Liquiritigenin |
| Isoliquiritigenin |
| QR CQ DV1U1R DQ &&E Form |
| ISL |
| (2E)-1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one |
| gu17 |
| 2-Propen-1-one, 1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)-, (2E)- |
| MFCD00075907 |
| (2E)-1-(2,4-Dihydroxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one |
| (E)-1-(2,4-Dihydroxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one |
| ISOLIQUIRTIGENIN |
| 4,2',4'-TRIHYDROXYCHALCONE |
| Isqliquiritigenin |
| 2',4',4-TRIHYDROXYCHALCONE |
| (E)-1-(2,4-Dihydroxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one |
| 2',4,4'-Trihydroxychalcone |
 CAS#:89-84-9
CAS#:89-84-9 CAS#:123-08-0
CAS#:123-08-0 CAS#:74189-56-3
CAS#:74189-56-3 CAS#:6515-21-5
CAS#:6515-21-5 CAS#:6515-05-5
CAS#:6515-05-5 CAS#:100-52-7
CAS#:100-52-7 CAS#:578-86-9
CAS#:578-86-9![6-hydroxy-2-[hydroxy-(4-hydroxyphenyl)methyl]-1-benzofuran-3-one structure](https://image.chemsrc.com/caspic/457/14796-48-6.png) CAS#:14796-48-6
CAS#:14796-48-6 CAS#:89-86-1
CAS#:89-86-1 CAS#:2034-65-3
CAS#:2034-65-3 CAS#:111915-65-2
CAS#:111915-65-2![(2,4-dihydroxy-phenyl)-[4-(4-hydroxy-phenyl)-[1,2]dioxetan-3-yl]-methanone structure](https://image.chemsrc.com/caspic/147/61443-82-1.png) CAS#:61443-82-1
CAS#:61443-82-1 CAS#:85359-61-1
CAS#:85359-61-1 CAS#:64687-96-3
CAS#:64687-96-3
