Xanthine amine congener
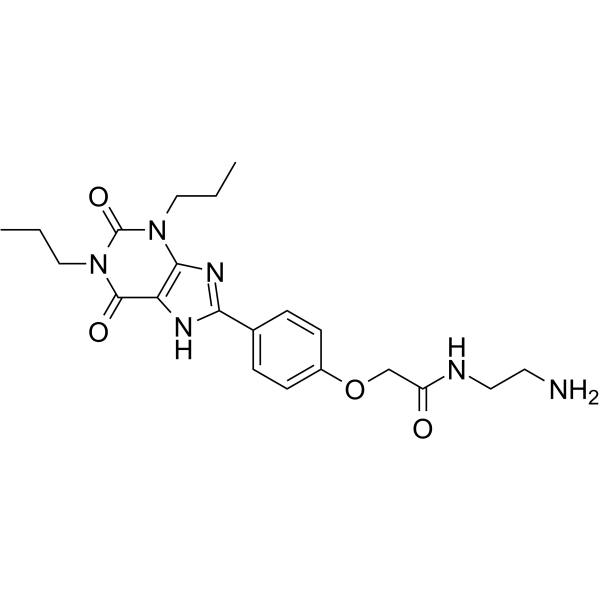
Xanthine amine congener structure
|
Common Name | Xanthine amine congener | ||
|---|---|---|---|---|
| CAS Number | 96865-92-8 | Molecular Weight | 428.48 | |
| Density | 1.262g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C21H28N6O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Xanthine amine congenerXanthine amine congener is a non-selective adenosine receptor antagonist[1]. Xanthine amine congener induces convulsions in mice[2]. |
| Name | N-(2-aminoethyl)-2-[4-(2,6-dioxo-1,3-dipropyl-7H-purin-8-yl)phenoxy]acetamide |
|---|---|
| Synonym | More Synonyms |
| Description | Xanthine amine congener is a non-selective adenosine receptor antagonist[1]. Xanthine amine congener induces convulsions in mice[2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.262g/cm3 |
|---|---|
| Molecular Formula | C21H28N6O4 |
| Molecular Weight | 428.48 |
| Exact Mass | 428.21700 |
| PSA | 137.03000 |
| LogP | 1.91820 |
| Index of Refraction | 1.586 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Chaperoning of the A1-adenosine receptor by endogenous adenosine - an extension of the retaliatory metabolite concept.
Mol. Pharmacol. 87(1) , 39-51, (2015) Cell-permeable orthosteric ligands can assist folding of G protein-coupled receptors in the endoplasmic reticulum (ER); this pharmacochaperoning translates into increased cell surface levels of recept... |
|
|
Allosteric interactions across native adenosine-A3 receptor homodimers: quantification using single-cell ligand-binding kinetics.
FASEB J. 25(10) , 3465-76, (2011) A growing awareness indicates that many G-protein-coupled receptors (GPCRs) exist as homodimers, but the extent of the cooperativity across the dimer interface has been largely unexplored. Here, measu... |
|
|
Hyperactivation of D1 and A2A receptors contributes to cognitive dysfunction in Huntington's disease.
Neurobiol. Dis. 74 , 41-57, (2015) Stimulation of dopamine D1 receptor (D1R) and adenosine A2A receptor (A2AR) increases cAMP-dependent protein kinase (PKA) activity in the brain. In Huntington's disease, by essentially unknown mechani... |
| Papaxac |
| XAC |
| Lopac-X-103 |
| xanthine amine congener |
| Xanthine amine congener xac |