6-氟吲哚
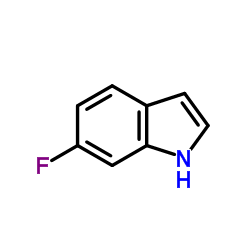
6-氟吲哚结构式

|
常用名 | 6-氟吲哚 | 英文名 | 6-fluoroindole |
|---|---|---|---|---|
| CAS号 | 399-51-9 | 分子量 | 135.14 | |
| 密度 | 1.3±0.1 g/cm3 | 沸点 | 258.0±13.0 °C at 760 mmHg | |
| 分子式 | C8H6FN | 熔点 | 72-76 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 109.9±19.8 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators.
J. Med. Chem. 54 , 5320, (2011) Tryptophan catabolism mediated by indoleamine 2,3-dioxygenase (IDO) is an important mechanism of peripheral immune tolerance contributing to tumoral immune resistance. IDO inhibition is thus an active area of research in drug development. Recently, our group ... |
|
|
Ionization potentials of fluoroindoles and the origin of nonexponential tryptophan fluorescence decay in proteins.
J. Am. Chem. Soc. 127(11) , 4104-13, (2005) This work reports an explanation for the unusual monoexponential fluorescence decay of 5-fluorotryptophan (5FTrp) in single-Trp mutant proteins [Broos, J.; Maddalena, F.; Hesp, B. H. J. Am. Chem. Soc. 2004, 126, 22-23] and substantially clarifies the origin o... |
|
|
Discovery of novel N-β-D-xylosylindole derivatives as sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes.
J. Med. Chem. 54 , 166, (2011) A novel series of N-linked β-D-xylosides were synthesized and evaluated for inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) in a cell-based assay. Of these, the 4-chloro-3-(4-cyclopropylbenzyl)-1-(β-D-xylopyranosyl)-1H-indole 19m ... |
|
|
Synthesis and evaluation of 1-(1H-indol-3-yl)ethanamine derivatives as new antibacterial agents.
Bioorg. Med. Chem. 19 , 3204, (2011) A collection of 3-substituted indole derivatives was prepared using nucleophilic addition of indoles to nitrones. The compounds were then tested for their antibacterial activity against almost thirty bacterial strains representative of common human pathogens.... |
|
|
3-(2-(3-Pyridinyl)thiazolidin-4-oyl)indoles, a novel series of platelet activating factor antagonists.
J. Med. Chem. 37 , 2011, (1994) (2RS,4R)-3-(2-(3-Pyridinyl)thiazolidin-4-oyl)indoles represent a new class of potent, orally active antagonists of platelet activating factor (PAF). The compounds were prepared by acylation of the magnesium or zinc salts of substituted indoles with (2RS,4R)-2... |
|
|
New indole derivatives as potent and selective serotonin uptake inhibitors.
J. Med. Chem. 36 , 1194, (1993) A series of new indole derivatives (2-28) has been prepared in the search for novel 5-HT uptake inhibitors. These compounds were obtained by the condensation of N-(chloroalkyl) naphthalenesultam derivatives with the appropriate amine in presence of a base, at... |
|
|
Synthesis and structure-affinity relationships of novel small molecule natural product derivatives capable of discriminating between serotonin 5-HT1A, 5-HT2A, 5-HT2C receptor subtypes.
Bioorg. Med. Chem. 18 , 4783, (2010) Efforts to develop ligands that distinguish between clinically relevant 5-HT2A and 5-HT2C serotonin receptor subtypes have been challenging, because their sequences have high homology. Previous studies reported that a novel aplysinopsin belonging to a chemica... |
|
|
Conformationally restricted homotryptamines. Part 7: 3-cis-(3-aminocyclopentyl)indoles as potent selective serotonin reuptake inhibitors.
J. Med. Chem. 53 , 7564, (2010) A series of conformationally restricted homotryptamines has been synthesized and shown to be potent inhibitors of hSERT. Conformational restriction of the homotryptamine side chain was attained by the insertion of a cyclopentyl ring, with the indole ring and ... |
|
|
Syntheses of halogen derivatives of L-tryptophan, L-tyrosine and L-phenylalanine labeled with hydrogen isotopes.
J. Labelled Comp. Radiopharm. 59 , 08-Apr, (2016) Halogenated, labeled with tritium and doubly with deuterium and tritium, derivatives of L-tryptophan, i.e. 5'-bromo-[2-(3)H]-, 5'-bromo-[2-(2)H/(3)H]-, 5'-fluoro-[2-(3)H]-5'-fluoro-[2-(2)H/(3)H]-, 6'-fluoro-[2-(3)H]-, 6'-fluoro-[2-(2)H/(3)H]-L-tryptophan, as ... |
|
|
Meanwell, N., A.; et al.
Bioorg. Med. Chem. Lett. 20 , 1460, (2010)
|