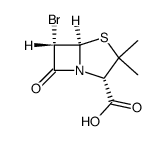
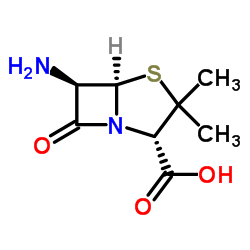
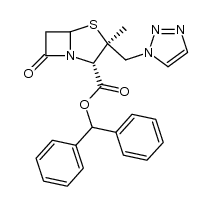
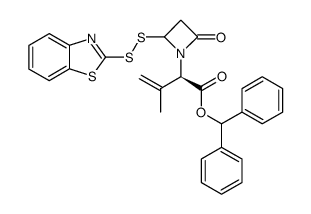
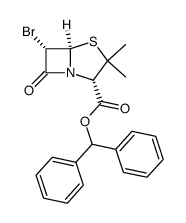
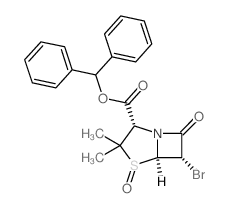
89786-04-9
| 中文名 | 他唑巴坦 |
|---|---|
| 英文名 | tazobactam |
| 中文别名 |
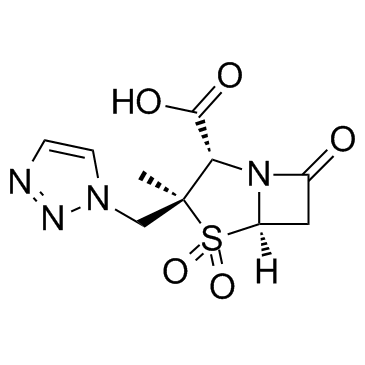
2α-甲基-2β-(1,2,3-三氮唑-1-基)甲基青霉烷砜-3α-羧酸
三唑巴坦 三唑烷砜 |
| 英文别名 |
MFCD00867002
YTR 830H Tazobactam [2S-(2a,3b,5a)]-3-Methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide T45 ANV ESWTJ F1 GVQ F1- AT5NNNJ &&(2S,3S,5R)- Form (2S,3S,5R)-3-Methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide UNII-SE10G96M8W TAZOBACTANACID YTR-830H 2b-[(1,2,3-triazol-1-yl)methyl]-2a-methylpenam-3a-carboxylic acid 1,1-dioxide Tazobactan acid 2-METHANESULFONYL-5-(TRIFLUOROMETHYL)-1,3,4-THIADIAZOLE |
| 密度 | 1.9±0.1 g/cm3 |
|---|---|
| 沸点 | 707.1±70.0 °C at 760 mmHg |
| 熔点 | 172 °C(dec.) |
| 分子式 | C10H12N4O5S |
| 分子量 | 300.291 |
| 闪点 | 381.4±35.7 °C |
| 精确质量 | 300.052826 |
| PSA | 130.84000 |
| LogP | -1.70 |
| 外观性状 | 白色或灰白色粉末 |
| 蒸汽压 | 0.0±2.4 mmHg at 25°C |
| 折射率 | 1.818 |
| 储存条件 | Store at?0-5°C |
| 水溶解性 | 水溶性:实际上不溶;可溶于:二甲基甲酰胺;极微溶:乙醇,甲醇 |
| 分子结构 | 1、 摩尔折射率:49.42 2、 摩尔体积(cm3/mol):143.4 3、 等张比容(90.2K):416.9 4、 表面张力(dyne/cm):71.3 5、 极化率(10-24cm3):19.59 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):-2 2.氢键供体数量:1 3.氢键受体数量:7 4.可旋转化学键数量:3 5.互变异构体数量:2 6.拓扑分子极性表面积131 7.重原子数量:20 8.表面电荷:0 9.复杂度:573 10.同位素原子数量:0 11.确定原子立构中心数量:3 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1.性状:白色粉末状结晶 2.溶解性:几乎不溶于水 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 危害码 (欧洲) | Xi: Irritant; |
|---|---|
| 风险声明 (欧洲) | R36/37/38 |
| 安全声明 (欧洲) | S26-S36/37/39-S45 |
| 危险品运输编码 | UN 1770 8/PG 2 |
| WGK德国 | 3 |
| 包装等级 | II |
| 危险类别 | 8 |
| 海关编码 | 29036990 |
|
~% 
89786-04-9 |
| 文献:Synthesis, , # 3 art. no. F11904SS, p. 442 - 446 |
|
~% 
89786-04-9 |
| 文献:Synthesis, , # 3 art. no. F11904SS, p. 442 - 446 |
|
~% 
89786-04-9 |
| 文献:Synthesis, , # 3 art. no. F11904SS, p. 442 - 446 |
|
~% 
89786-04-9 |
| 文献:Synthesis, , # 3 art. no. F11904SS, p. 442 - 446 |
|
~% 
89786-04-9 |
| 文献:Synthesis, , # 3 art. no. F11904SS, p. 442 - 446 |
|
~% 
89786-04-9 |
| 文献:Synthesis, , # 3 art. no. F11904SS, p. 442 - 446 |
|
~% 
89786-04-9 |
| 文献:Synthesis, , # 3 art. no. F11904SS, p. 442 - 446 |
| 上游产品 6 | |
|---|---|
| 下游产品 2 | |
| 海关编码 | 29036990 |
|---|