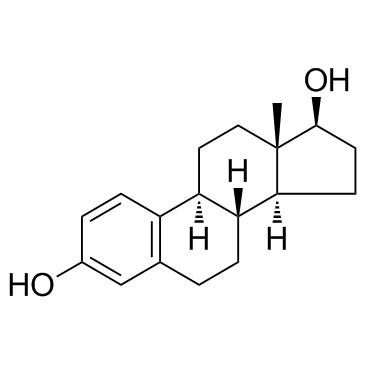
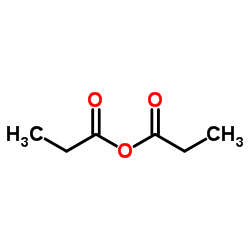
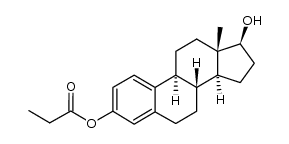
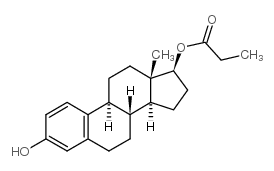
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KG4725000
-
CHEMICAL NAME :
-
Estradiol, dipropionate
-
CAS REGISTRY NUMBER :
-
113-38-2
-
BEILSTEIN REFERENCE NO. :
-
3223915
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
32
-
MOLECULAR FORMULA :
-
C24-H32-O4
-
MOLECULAR WEIGHT :
-
384.56
-
WISWESSER LINE NOTATION :
-
L E5 B666TTT&J E1 FOV2 OOV2
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,175,1990 ** TUMORIGENIC DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
40 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Skin and Appendages - tumors
-
REFERENCE :
-
CNREA8 Cancer Research. (Public Ledger Building, Suit 816, 6th & Chestnut Sts., Philadelphia, PA 19106) V.1- 1941- Volume(issue)/page/year: 2,632,1942
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
36 mg/kg/36W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Blood - leukemia Blood - lymphoma, including Hodgkin's disease
-
REFERENCE :
-
YJBMAU Yale Journal of Biology and Medicine. (333 Cedar St., New Haven, CT 06510) V.1- 1928- Volume(issue)/page/year: 12,213,1939
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
2200 ug/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Tumorigenic - tumors at site of application
-
REFERENCE :
-
RCBIAS Revue Canadienne de Biologie. (Montreal, Quebec, Canada) V.1-40, 1942-81. Volume(issue)/page/year: 3,108,1944
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3000 mg/kg/25W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Skin and Appendages - tumors
-
REFERENCE :
-
JPBAA7 Journal of Pathology and Bacteriology. (London, UK) V.1-96, 1892-1968. For publisher information, see JPTLAS. Volume(issue)/page/year: 51,9,1940
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
54 mg/kg/27W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Blood - leukemia Skin and Appendages - tumors
-
REFERENCE :
-
YJBMAU Yale Journal of Biology and Medicine. (333 Cedar St., New Haven, CT 06510) V.1- 1928- Volume(issue)/page/year: 12,213,1939 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
12500 ug/kg
-
SEX/DURATION :
-
female 16-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 36,1069,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages
-
REFERENCE :
-
JOPHAN Journal de Physiologie (Paris). (SPPIF, B.P.22, F-41353 Vineuil, France) V.39- 1946- Volume(issue)/page/year: 55,237,1963
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
45500 ng/kg
-
SEX/DURATION :
-
female 28 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
REFERENCE :
-
IJEBA6 Indian Journal of Experimental Biology. (Publications & Information Directorate, CSIR, Hillside Rd., New Delhi 110 012, India) V.1- 1963- Volume(issue)/page/year: 11,1,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
348 ug/kg
-
SEX/DURATION :
-
male 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands Reproductive - Paternal Effects - other effects on male
-
REFERENCE :
-
IJEBA6 Indian Journal of Experimental Biology. (Publications & Information Directorate, CSIR, Hillside Rd., New Delhi 110 012, India) V.1- 1963- Volume(issue)/page/year: 27,324,1989
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
2500 ng/kg
-
SEX/DURATION :
-
female 5 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
REFERENCE :
-
IJEBA6 Indian Journal of Experimental Biology. (Publications & Information Directorate, CSIR, Hillside Rd., New Delhi 110 012, India) V.1- 1963- Volume(issue)/page/year: 16,648,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
300 ug/kg
-
SEX/DURATION :
-
male 15 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 1,373,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
600 ug/kg
-
SEX/DURATION :
-
male 30 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 1,373,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
12 ug/kg
-
SEX/DURATION :
-
male 30 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 1,373,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
60 ug/kg
-
SEX/DURATION :
-
male 15 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 1,373,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages
-
REFERENCE :
-
AAMMAU Archives d'Anatomie Microscopique et de Morphologie Experimentale. (SPPIF, B.P.22, F-41353 Vineuil, France) V.36-76, 1947-1987. For publisher information, see BSTME2. Volume(issue)/page/year: 60,147,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
1 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord)
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 164,779,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 164,784,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 159,2357,1965
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
8 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 158,2321,1964
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 149,1233,1955
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
24 ug/kg
-
SEX/DURATION :
-
female 3 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
REFERENCE :
-
IJEBA6 Indian Journal of Experimental Biology. (Publications & Information Directorate, CSIR, Hillside Rd., New Delhi 110 012, India) V.1- 1963- Volume(issue)/page/year: 16,419,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
4 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 160,976,1966
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
4 mg/kg
-
SEX/DURATION :
-
female 13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages
-
REFERENCE :
-
AIPAAV Annales de l'Institut Pasteur (Paris). (Paris, France) V.1-123, 1887-1972. For publisher information, see ANMBCM. Volume(issue)/page/year: 90,187,1956
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord)
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 164,779,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
4 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 164,784,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
4 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 159,2357,1965
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
JOPHAN Journal de Physiologie (Paris). (SPPIF, B.P.22, F-41353 Vineuil, France) V.39- 1946- Volume(issue)/page/year: 57,634,1965
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
10 ug/kg
-
SEX/DURATION :
-
female 1 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 6,207,1972 *** REVIEWS *** IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 21,279,1979 TOXICOLOGY REVIEW ACRSAJ Advances in Cancer Research. (Academic Press, Inc., 465 S. Lincoln Dr., Troy, MO 63379) V.1- 1953- Volume(issue)/page/year: 1,173,1953
|