达诺沙星
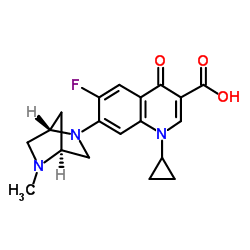
达诺沙星结构式

|
常用名 | 达诺沙星 | 英文名 | Danofloxacine |
|---|---|---|---|---|
| CAS号 | 112398-08-0 | 分子量 | 357.379 | |
| 密度 | 1.5±0.1 g/cm3 | 沸点 | 569.3±50.0 °C at 760 mmHg | |
| 分子式 | C19H20FN3O3 | 熔点 | 268-272ºC | |
| MSDS | 中文版 美版 | 闪点 | 298.1±30.1 °C |
达诺沙星用途Danofloxacin 是一种具有口服活性第的三代氟喹诺酮抗菌剂,对大多数革兰氏阴性和革兰氏阳性细菌,支原体和衣原体物种具有广谱的活性,并通过抑制细菌的 DNA 回转酶发挥抗菌作用。Danofloxacinh 可用于牛,猪和鸡呼吸道疾病的研究。 |
| 中文名 | 达氟沙星 |
|---|---|
| 英文名 | Danofloxacin |
| 中文别名 | 达诺沙星 |
| 英文别名 | 更多 |
| 描述 | Danofloxacin 是一种具有口服活性第的三代氟喹诺酮抗菌剂,对大多数革兰氏阴性和革兰氏阳性细菌,支原体和衣原体物种具有广谱的活性,并通过抑制细菌的 DNA 回转酶发挥抗菌作用。Danofloxacinh 可用于牛,猪和鸡呼吸道疾病的研究。 |
|---|---|
| 相关类别 | |
| 参考文献 |
| 密度 | 1.5±0.1 g/cm3 |
|---|---|
| 沸点 | 569.3±50.0 °C at 760 mmHg |
| 熔点 | 268-272ºC |
| 分子式 | C19H20FN3O3 |
| 分子量 | 357.379 |
| 闪点 | 298.1±30.1 °C |
| 精确质量 | 357.148865 |
| PSA | 65.78000 |
| LogP | 1.20 |
| InChIKey | QMLVECGLEOSESV-RYUDHWBXSA-N |
| SMILES | C[NH+]1CC2CC1CN2c1cc2c(cc1F)c(=O)c(C(=O)[O-])cn2C1CC1 |
| 外观性状 | 无色粉末 |
| 蒸汽压 | 0.0±1.6 mmHg at 25°C |
| 折射率 | 1.679 |
| 储存条件 | 2-8℃,充氩保存 |
| 水溶解性 | Soluble in water, acetic acid and DMSO |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危害码 (欧洲) | Xn,Xi |
| 风险声明 (欧洲) | 20/21/22-36/37/38 |
| 安全声明 (欧洲) | 22-24/25 |
| 危险品运输编码 | NONH for all modes of transport |
| 达诺沙星上游产品 1 | |
|---|---|
| 达诺沙星下游产品 0 | |
|
Multiresidue determination of fluoroquinolones in poultry muscle and kidney according to the regulation 2002/657/EC. A systematic comparison of two different approaches: Liquid chromatography coupled to high-resolution mass spectrometry or tandem mass spectrometry.
J. Chromatogr. A. 1379 , 83-91, (2015) This work involved the optimization and validation of two methods according to the Commission Decision 2002/657/EC directives for determining fluoroquinolones residues in samples of poultry muscle and... |
|
|
Simultaneous determination of 38 veterinary antibiotic residues in raw milk by UPLC-MS/MS.
Food Chem. 181 , 119-26, (2015) A selective and rapid method has been developed to determine, simultaneously, 38 veterinary antibiotic residues in raw milk by ultra-high-performance liquid chromatography-tandem mass spectrometry (UP... |
|
|
Decay mechanisms of protonated 4-quinolone antibiotics after electrospray ionization and ion activation.
J. Am. Soc. Mass Spectrom. 25(11) , 1974-86, (2014) This study presents a detailed experimental investigation of charge isomers of protonated 4-quinolone antibiotics molecules formed during electrospray ionization (ESI) with proposed dissociation mecha... |
| 1-Cyclopropyl-6-fluoro-7-[(1S,4S)-5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl]-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid |
| danofloxacin |
| 1-Cyclopropyl-6-fluoro-7-[(1S,4S)-5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid |
| (1S)-1-Cyclopropyl-6-fluoro-1,4-dihydro-7-(5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl)-4-oxo-3-quinolinecarboxylic acid |

![2-甲基-2,5-二氮杂双环[2.2.1]庚烷二盐酸盐结构式](https://image.chemsrc.com/caspic/086/52321-26-3.png)






