Copper cyanide
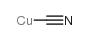
Copper cyanide structure
|
Common Name | Copper cyanide | ||
|---|---|---|---|---|
| CAS Number | 544-92-3 | Molecular Weight | 89.56340 | |
| Density | 2.92 g/mL at 25 °C(lit.) | Boiling Point | N/A | |
| Molecular Formula | CCuN | Melting Point | 474 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 140°C(lit.) | |
| Symbol |

GHS06, GHS09 |
Signal Word | Danger | |
|
Iron(VI) and iron(V) oxidation of copper(I) cyanide.
Environ. Sci. Technol. 39(10) , 3849-54, (2005) Copper(Il) cyanide (Cu(CN)4(3-)) in the gold mine industry presentsthe biggest concern in cyanide management because it is much more stable than free cyanide. Cu(CN)4(3-) is highlytoxic to aquatic life; therefore, environmentally friendly techniques are requi... |
|
|
The first precise molecular structure of a monomeric transition metal cyanide, copper(I) cyanide.
J. Am. Chem. Soc. 124(20) , 5895-901, (2002) Copper(I) cyanide is an important reagent in organic, organometallic, and supramolecular chemistry because of both the copper center and the versatile cyanide ligand. Solid-phase CuCN and many of its derivatives show oligomeric or polymeric structures, a trai... |
|
|
Crystal Structures of a Series of Complexes Produced by Reaction of Copper(I) Cyanide with Diamines.
Inorg. Chem. 38 , 984, (1999) A new synthetic procedure developed recently in our laboratories has made possible the synthesis of variety of new complexes of CuCN with diamines. Synthesis was effected by adding the ligand to a solution of CuCN in aqueous sodium thiosulfate. This procedure... |
|
|
Crystal Structures of a Family of New Copper(I) Cyanide Complexes of Thiourea and Substituted Thioureas.
Inorg. Chem. 35(11) , 3145-3153, (1996) The syntheses and crystal structures of the first cyanide, sulfur mixed ligand copper(I) complexes are reported. The first complex of the family was discovered when (CuCN)(3)(C(6)H(12)N(4))(2) (1) (C(6)H(12)N(4) = hexamethylenetetramine) was treated with aque... |