Pinocembrin-7-methyl ether
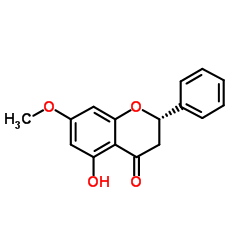
Pinocembrin-7-methyl ether structure
|
Common Name | Pinocembrin-7-methyl ether | ||
|---|---|---|---|---|
| CAS Number | 480-37-5 | Molecular Weight | 270.280 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 494.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C16H14O4 | Melting Point | 100ºC | |
| MSDS | Chinese USA | Flash Point | 188.8±22.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Phloroglucinols from anti-microbial deposit-resins of Australian stingless bees (Tetragonula carbonaria).
Phytother Res. 29(1) , 48-58, (2015) Stingless bees accumulate deposits of plant resins that are mixed with beeswax to produce propolis. Previous studies have reported anti-microbial constituents of stingless bee (Tetragonula carbonaria) propolis from East Australia, but several components remai... |
|
|
Study on the constituents of Mexican propolis and their cytotoxic activity against PANC-1 human pancreatic cancer cells.
J. Nat. Prod. 73 , 623-7, (2010) Three new flavonoids, (2R,3R)-3,5-dihydroxy-7-methoxyflavanone 3-(2-methyl)butyrate (1), (7''R)-8-[1-(4'-hydroxy-3'-methoxyphenyl)prop-2-en-1-yl]chrysin (2), and (7''R)-8-[1-(4'-hydroxy-3'-methoxyphenyl)prop-2-en-1-yl]galangin (3), together with 41 known comp... |
|
|
Cytotoxic flavonoids from the leaves of Cryptocarya chinensis.
J. Nat. Prod. 73 , 1470-5, (2010) Bioassay-guided fractionation led to the isolation of six new tetrahydroflavanones, cryptochinones A-F (1-6), from the neutral CHCl(3) fraction of Cryptocarya chinensis leaves, together with 14 known compounds (7-20). The structures of these new compounds wer... |
|
|
Structure-activity relationships of flavonoids as inhibitors of breast cancer resistance protein (BCRP).
Bioorg. Med. Chem. 19 , 2090-102, (2011) Flavonoids are an interesting group of natural products ubiquitously present in human diet. Their consumption has been associated with various and differing beneficial health effects. However, several flavonoids have been reported to inhibit the breast cancer... |
|
|
Antimalarials from nature.
Bioorg. Med. Chem. 17 , 3229-56, (2009) Malaria is a major public health problem mainly due to the development of resistance by the most lethal causative parasitic species, Plasmodium falciparum to the mainstay drugs like chloroquine. New drugs with unique structures and mechanism of action are urg... |
|
|
Effects of pinostrobin on estrogen metabolism and estrogen receptor transactivation.
Cancer Lett. 156 , 37-44, (2000) The interaction between the estrogen receptor and 5-hydroxy-7-methoxyflavanone (pinostrobin) was studied in the presence or absence of estradiol or dehydroepiandrosterone sulfate (DHEAS), respectively, using a stably transfected human breast cancer cell line ... |
|
|
Bioactive secondary metabolites from Boesenbergia pandurata of Myanmar and their preferential cytotoxicity against human pancreatic cancer PANC-1 cell line in nutrient-deprived medium.
J. Nat. Prod. 70 , 1582-7, (2007) The chloroform extract of rhizomes of Boesenbergia pandurata demonstrated marked preferential cytotoxicity against human pancreatic PANC-1 cancer cells in nutrient-deprived medium. Bioactivity-directed investigation of this extract yielded four new secondary ... |
|
|
Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, Boesenbergia rotunda (L.), towards dengue-2 virus NS3 protease.
Bioorg. Med. Chem. Lett. 16 , 3337-40, (2006) Boesenbergia rotunda (L.) cyclohexenyl chalcone derivatives, 4-hydroxypanduratin A and panduratin A, showed good competitive inhibitory activities towards dengue 2 virus NS3 protease with the Ki values of 21 and 25 microM, respectively, whilst those of pinost... |
|
|
Cytotoxicity against KB and NCI-H187 cell lines of modified flavonoids from Kaempferia parviflora.
Bioorg. Med. Chem. Lett. 20 , 2821-3, (2010) Flavones 1-4 isolated from Kaempferia parviflora were used for structural modification. Sixteen flavonoid derivatives, including four new derivatives, were synthesized and evaluated for cytotoxicity against KB and NCI-H187 cell lines. Flavanones 2a-4a demonst... |
|
|
Efficient microwave-assisted prenylation of pinostrobin and biological evaluation of its derivatives as antitumor agents.
Bioorg. Med. Chem. Lett. 20 , 2086-9, (2010) Pinostrobin (5-hydroxy-7-methoxyflavanone) obtained in relatively large amounts from fingerroot (Boesenbergia pandurata) was converted to its C-6 and C-8 prenylated derivatives. The Mitsunobu reaction, europium(III)-catalyzed Claisen-Cope rearrangement, and C... |