| Structure | Name/CAS No. | Articles |
|---|---|---|
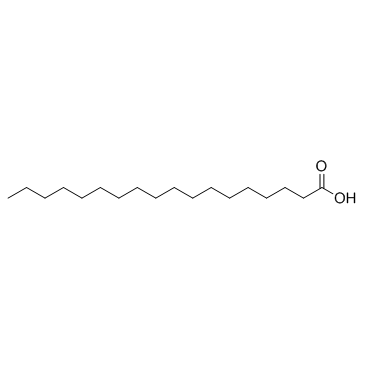 |
stearic acid
CAS:57-11-4 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
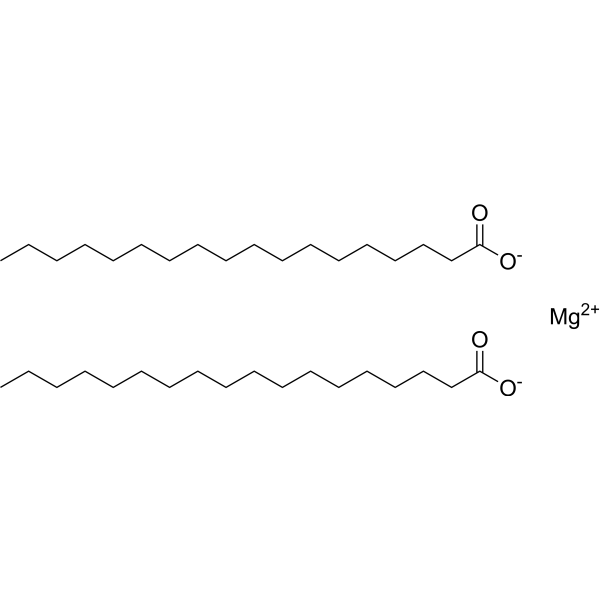 |
Magnesium stearate
CAS:557-04-0 |
|
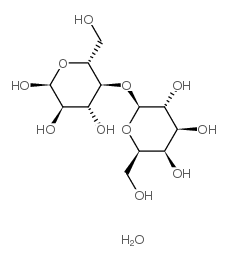 |
D-(+)-Lactose Monohydrate
CAS:64044-51-5 |
|
 |
Lactose
CAS:63-42-3 |
|
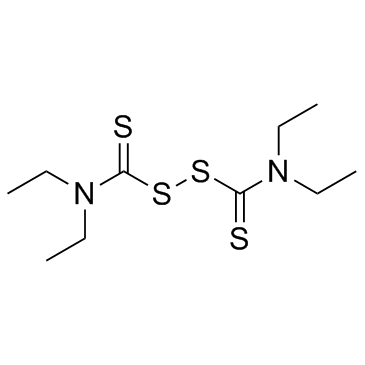 |
Disulfiram
CAS:97-77-8 |