81093-37-0
| Name | pravastatin |
|---|---|
| Synonyms |
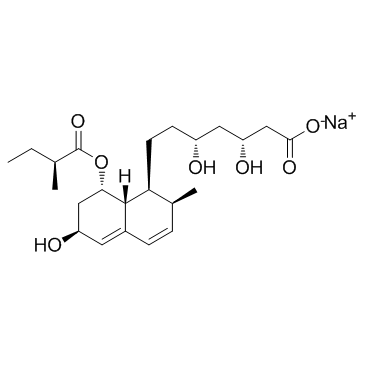
(3R,5R)-3,5-Dihydroxy-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-{[(2S)-2-methylbutanoyl]oxy}-1,2,6,7,8,8a-hexahydro-1-naphthalenyl]heptanoic acid
[14C]-Pravastatin Oliprevin MFCD00887601 Pravastatina (3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-[(2S)-2-methylbutanoyl]oxy-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoic acid Pravachol Pravastatinum (3R,5R)-3,5-Dihydroxy-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-{[(2S)-2-methylbutanoyl]oxy}-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]heptanoic acid Pravastatin [3H]-Pravastatin Pravastatine [French] Pravastatina [Spanish] (3R,5R)-3,5-dihydroxy-7-{(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-[(2S)-2-methylbutanoyloxy]-1,2,6,7,8,8a-hexahydronaphthalen-1-yl}heptanoic acid Pravastatine Pravastatinum [Latin] Eptastatin |
| Description | Pravastatin is an HMG-CoA reductase inhibitor against sterol synthesis with IC50 of 5.6 μM.Target: HMG-CoA reductasePravastatin (marketed as Pravachol or Selektine) is a member of the drug class of statins, used in combination with diet, exercise, and weight-loss for lowering cholesterol and preventing cardiovascular disease.Pravastatin is primarily used for the treatment of dyslipidemia and the prevention of cardiovascular disease. It is recommended to be used only after other measures such as diet, exercise, and weight reduction have not improved cholesterol levels.The evidence for the use of pravastatin is generally weaker than for other statins. The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT), failed to demonstrate a difference in all-cause mortality or nonfatal myocardial infarction/fatal coronary heart disease rates between patients receiving pravastatin 40mg daily (a common starting dose) and those receiving usual care. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 634.5±55.0 °C at 760 mmHg |
| Melting Point | 171.2-173ºC |
| Molecular Formula | C23H36O7 |
| Molecular Weight | 424.53 |
| Flash Point | 213.2±25.0 °C |
| PSA | 127.12000 |
| LogP | 1.35 |
| Vapour Pressure | 0.0±4.2 mmHg at 25°C |
| Index of Refraction | 1.555 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: 19 mg/mL |
| Hazard Codes | F: Flammable;C: Corrosive; |
|---|---|
| Risk Phrases | R11;R34 |
| Safety Phrases | S16-S26-S36/37/39-S45 |
| WGK Germany | 2 |
| RTECS | QJ7185000 |
|
~% 
81093-37-0 |
| Literature: WO2005/121062 A2, ; Page/Page column 6 ; |
|
~88% 
81093-37-0 |
| Literature: Biocon Limited; Tiwari, Sanjay; Patale, Mukesh Babuappa; Garg, Saurabh; Garg, Mayank Kumar; Joshi, Sulekha; Kumar, Chittnalli Ramegowda Naveen; Kumar, Bimal; Goel, Anuj; Iyer, Harish Patent: US2014/141467 A1, 2014 ; Location in patent: Page/Page column ; |
|
~% 
81093-37-0 |
| Literature: Journal of Antibiotics, , vol. 50, # 12 p. 1032 - 1035 |
| Precursor 2 | |
|---|---|
| DownStream 2 | |