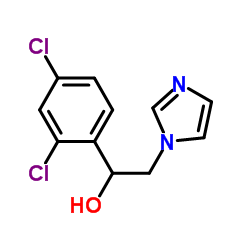
99592-32-2
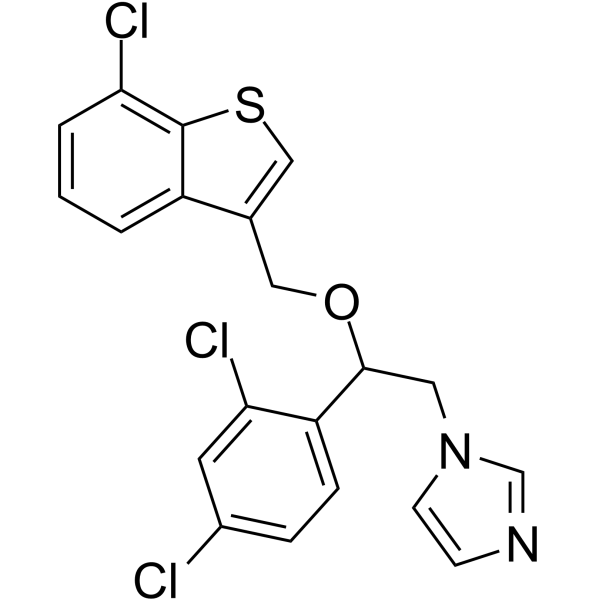
| Name | 1-[2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole |
|---|---|
| Synonyms |
Sertaconazolum
Sertaconazol 1-{2-[(7-Chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole T56 BSJ IG D1OYR BG DG&1- AT5N CNJ Sertaconazole (INN) MFCD00868881 Sertaconazolum [Latin] sertaconazole Ertaczo Sertaconazol [Spanish] 1-{2-[(7-chloro-3-benzo[b]thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole 1-[2-(7-chlorobenzo[b]thiophene-3-yl-methoxy)-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole 7-Chloro-3-[1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethoxy-methyl]benzo[b]thiophene 1-[2-[(7-chlorobenzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)-ethyl]imidazole (±)-1-[2,4-Dichloro-β-[(7-chlorobenzo[b]thien-3-yl)methoxy]phenethyl]imidazole FI-7045 7-chloro-3-[1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethoxymethyl]benzo[b]thiophene 1-{2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}imidazole |
| Description | Sertaconazole (FI7056 free base) is a broad-spectrum topical antifungal agent, exhibits anti-inflammatory activity via activation of a p38-COX-2-PGE2 pathway. Sertaconazole is also a microtubule inhibitor, shows antiproliferative effect, induces apoptosis and autophagy, and can also inhibit the migration of cells[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Sertaconazole (0.03-40 µg/mL; 24 h) inhibits 150 strains of yeasts which includes six Candida species with arithmetic mean MIC of 0.77 µg/mL[1]. Sertaconazole (1 µg/mL; 5, 10, 30, 60 min) activates p38 MAP kinase in a time-dependent manner[2]. Sertaconazole (1, 2 µg/mL; 6, 8, or 24 h) increases a twofold release of PGE2 via COX-2 in keratinocytes, which is dependent on p38 activation[2]. Cetaconazole (10, 20, 30, 40 µM; 24 h) induces strong mitotic arrest by depolymerizing interphase and spindle microtubules, thereby inducing chromosome aggregation defects and causing anti-proliferation effect[3]. Sertaconazole (20, 40 µM; 24 h) induces apoptosis through p53 pathway in HeLa cells[3]. Sertaconazole (20, 30 µM; 24, 48, and 72 h) inhibits the migration of HeLa cells in a concentration-dependent manner[3]. Sertaconazole (15, 30 µM; 24 h) induces autophagy in A549, H460 cells[4]. Cell Viability Assay[1] Cell Line: C. albicans, C. guilliermondii, C. krusei, C. parapsilosi, C. tropicalis, C. glabrata Concentration: 0.03-40 µg/m Incubation Time: 24 h Result: Againsted 150 strains of yeasts (six Candida species) which included C. albicans, C. guilliermondii, C. krusei, C. parapsilosi, C. tropicalis, C. glabrata species with arithmetic mean MIC values of 1.02, 0.51, 0.38, 0.31, 1.67 and 0.78 µg/mL, respectively. Western Blot Analysis[2] Cell Line: HaCaT cells Concentration: 1 µg/mL Incubation Time: 5, 10, 30, 60 min Result: Showed activity of activating p38 MAP kinase and Hsp27 in a time-dependent manner. Western Blot Analysis[2] Cell Line: HaCaT cells Concentration: 1, 2 µg/mL Incubation Time: 6 or 8 h Result: Induced 50% expression of COX-2 and resulted in a twofold increased in PGE2 release. Western Blot Analysis[2] Cell Line: siRNA-transfected HaCaT cells (without p38 MAP kinase expression) Concentration: 1 µg/mL Incubation Time: 24 h Result: Mediated induction of PGE2 was dependent on p38 activation. Cell Proliferation Assay[3] Cell Line: HeLa, HEK-293, MCF-7, A549 cells Concentration: 0-100 µM Incubation Time: 24 h Result: Showed antiproliferation activity with IC50s of 38, 45.1, 41.5, and 40.8 μM for HeLa, HEK-293, A549, and MCF-7 cells, respectively. Exhibited mitotic block activity and induced cell death at concentration above 30 μM, but no significant increased in the number of mitotic cells. Depolymerized interphase and spindle microtubules inducing defect in chromosomal congression. Apoptosis Analysis[3] Cell Line: HeLa cells Concentration: 10, 20, 40 µM Incubation Time: 24 h Result: Induced approximately 5%, 10%, and 21% cells apoptotic at concentrations of 10, 20 and 40 μM, respectively. Western Blot Analysis[3] Cell Line: A549 cells Concentration: 20, 40 µM Incubation Time: 24 h Result: Induced apoptosis through p53 pathway that the expression of p53 from 30% to 50% and 95% and p21 from 11 to 39% and 40% respectively. Resulted in Noxa and Puma, two direct transcriptional targets of p53 to be overexpressed. Cell Migration Assay [3] Cell Line: HeLa cells Concentration: 20, 30 µM Incubation Time: 24, 48, and 72 h Result: Inhibited the migration of HeLa cells at concentrations lesser than its IC50, which in a concentration-dependent manner. Cell Autophagy Assay[4] Cell Line: A549, H460 cells Concentration: 15, 30 µM Incubation Time: 24 h Result: Increased endogenous LC3 puncta and LC3 intensity, which indicated induction of autophagy in A549 and H460 cells. |
| In Vivo | Sertaconazole (1% (w/v); apply to the left ear, once) suppresses of TPA-induced ear edema CD-1 mice[2]. Animal Model: CD-1 mice (TPA-induced ear edema model)[2]. Dosage: 1% (w/v) Administration: Apply to the left ear, once. Result: Exhibited a significant reduction of inflammation in mice by mediating PGE2 release. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 614.1±55.0 °C at 760 mmHg |
| Molecular Formula | C20H15Cl3N2OS |
| Molecular Weight | 437.770 |
| Flash Point | 325.2±31.5 °C |
| Exact Mass | 435.997070 |
| PSA | 55.29000 |
| LogP | 7.49 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.675 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| Safety Phrases | S22-S24/25 |
| WGK Germany | 2 |
| RTECS | KM6557000 |
|
~% 
99592-32-2 |
| Literature: European Journal of Medicinal Chemistry, , vol. 21, # 4 p. 329 - 332 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |