64567-60-8
| Name | 2-(2,6-dioxopiperidin-3-yl)-5-hydroxyisoindole-1,3-dione |
|---|---|
| Synonyms | 5-Hydroxythalidomide |
| Description | Thalidomide-5-OH is the Thalidomide-based cereblon ligand used in the recruitment of CRBN protein. Thalidomide-5-OH can be connected to the ligand for protein by a linker to form PROTACs[1]. |
|---|---|
| Related Catalog | |
| Target |
Cereblon |
| References |
| Density | 1.611g/cm3 |
|---|---|
| Boiling Point | 600ºC at 760mmHg |
| Molecular Formula | C13H10N2O5 |
| Molecular Weight | 274.22900 |
| Flash Point | 316.7ºC |
| Exact Mass | 274.05900 |
| PSA | 107.27000 |
| LogP | 0.00720 |
| Index of Refraction | 1.679 |
| Hazard Codes | Xi |
|---|
|
~59% 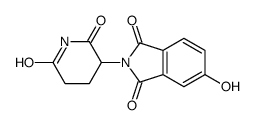
64567-60-8 |
| Literature: Nakamura, Takanori; Noguchi, Tomomi; Kobayashi, Hisayoshi; Miyachi, Hiroyuki; Hashimoto, Yuichi Chemical and Pharmaceutical Bulletin, 2006 , vol. 54, # 12 p. 1709 - 1714 |
|
~% 
64567-60-8 |
| Literature: Nakamura, Takanori; Noguchi, Tomomi; Kobayashi, Hisayoshi; Miyachi, Hiroyuki; Hashimoto, Yuichi Chemical and Pharmaceutical Bulletin, 2006 , vol. 54, # 12 p. 1709 - 1714 |
|
~% 
64567-60-8 |
| Literature: Nakamura, Takanori; Noguchi, Tomomi; Kobayashi, Hisayoshi; Miyachi, Hiroyuki; Hashimoto, Yuichi Chemical and Pharmaceutical Bulletin, 2006 , vol. 54, # 12 p. 1709 - 1714 |
|
~% 
64567-60-8 |
| Literature: Nakamura, Takanori; Noguchi, Tomomi; Kobayashi, Hisayoshi; Miyachi, Hiroyuki; Hashimoto, Yuichi Chemical and Pharmaceutical Bulletin, 2006 , vol. 54, # 12 p. 1709 - 1714 |
|
~% 
64567-60-8 |
| Literature: Nakamura, Takanori; Noguchi, Tomomi; Kobayashi, Hisayoshi; Miyachi, Hiroyuki; Hashimoto, Yuichi Chemical and Pharmaceutical Bulletin, 2006 , vol. 54, # 12 p. 1709 - 1714 |
|
~% 
64567-60-8 |
| Literature: Nakamura, Takanori; Noguchi, Tomomi; Kobayashi, Hisayoshi; Miyachi, Hiroyuki; Hashimoto, Yuichi Chemical and Pharmaceutical Bulletin, 2006 , vol. 54, # 12 p. 1709 - 1714 |
|
~% 
64567-60-8 |
| Literature: Nakamura, Takanori; Noguchi, Tomomi; Kobayashi, Hisayoshi; Miyachi, Hiroyuki; Hashimoto, Yuichi Chemical and Pharmaceutical Bulletin, 2006 , vol. 54, # 12 p. 1709 - 1714 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |