113712-98-4
| Name | Tenatoprazole |
|---|---|
| Synonyms |
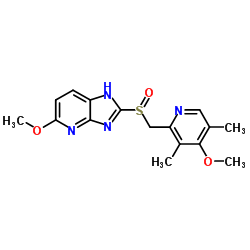
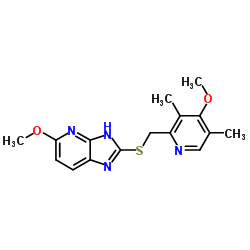
5-Methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-imidazo[4,5-b]pyridine
(±)-5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-imidazo[4,5-b]pyridine 5-Methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-3H-imidazo[4,5-b]pyridine tenatoprazole/tu-199 5-Methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-imidazo[4,5-b]pyridin Tenatoprazole 5-Methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl}-1H-imidazo[4,5-b]pyridine tenatoprazole monosodium |
| Description | Tenatoprazole (TU-199) is an orally active imidazopyridine-based proton pump inhibitor with a prolonged plasma half-life. Tenatoprazole inhibits hog gastric H+/K+-ATPase activity with an IC50 of 6.2 μM. Tenatoprazole blocks the interaction of ubiquitin with the ESCRT-1 factor Tsg101, inhibits production of several enveloped viruses, including EBV[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Tenatoprazole (TU-199) (0.1, 0.2, 0.4 mg/kg; oral; single; Heidenhain-pouch dogs) dose-dependently suppresses gastric acid secretion stimulated by histamine infusion[4]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 591.5±60.0 °C at 760 mmHg |
| Melting Point | 178-180°C |
| Molecular Formula | C16H18N4O3S |
| Molecular Weight | 346.40 |
| Flash Point | 311.5±32.9 °C |
| Exact Mass | 346.109955 |
| PSA | 109.20000 |
| LogP | 1.36 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.674 |
| Storage condition | −20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P301 + P312 + P330-P305 + P351 + P338 |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 26-36/37 |
| RIDADR | NONH for all modes of transport |
| Precursor 1 | |
|---|---|
| DownStream 0 | |