3785-90-8
| Name | 4-hydroxybenzylidenemalononitrile |
|---|---|
| Synonyms |
4-(HYDROXYBENZYLIDENE)-MALONONITRILE
4-Hydroxybenzylidenemalononitrile (4-Hydroxybenzylidene)malonitrile EINECS 223-253-0 2-[(4-hydroxyphenyl)methylidene]propanedinitrile MFCD00020189 |
| Description | Tyrphostin 8 is a tyrosine kinase, with an IC50 of 560 μM for EGFR kinase. Tyrphostin 8 is also a GTPase inhibitor. Tyrphostin 8 can inhibit the protein serine/threonine phosphatase calcineurin (IC50=21 μM)[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
EGFR:560 μM (IC50) calcineuin phosphatase:21 μM (IC50) |
| In Vitro | Tyrphostin 8 (10-100 μM; pretreated for 20 min) blocks the Carbachol-initiated PKCδ tyrosine phosphorylation and ERK1/2 activation in parotid acinar cells[1]. Tyrphostin 8 (10-100 μM) produces a rapid and large increase in the basal O2 consumption of parotid acinar[1]. Tyrphostin 8 (10-100 μM) reduces the parotid ATP content by ∼90% at the concentration of 100 μM[1]. Tyrphostin 8 increases apical-to-basolateral transport of insulin-transferrin conjugate by enhancing transferrin receptor-mediated transcytosis in filter-grown Caco-2 cell monolayer[2]. Western Blot Analysis[1] Cell Line: Parotid acinar cells Concentration: 10, 100 μM Incubation Time: Pretreated for 20 min Result: Reduced the increase in tyrosine phosphorylation of PKCδ initiated by carbachol. Reduced the activation of ERK1/2 by carbachol. |
| In Vivo | Tyrphostin 8 improves the glucose-lowering effect of Insulin-transferrin in Streptozotocin-induced diabetic rats[2]. |
| References |
| Density | 1.29 g/cm3 |
|---|---|
| Boiling Point | 354.3ºC at 760 mmHg |
| Melting Point | 186-189 °C |
| Molecular Formula | C10H6N2O |
| Molecular Weight | 170.17 |
| Flash Point | 168.1ºC |
| Exact Mass | 170.04800 |
| PSA | 67.81000 |
| LogP | 1.82276 |
| Index of Refraction | 1.653 |
| Storage condition | 2-8°C |
| Water Solubility | ethanol: 20 mg/mL, clear |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H319 |
| Precautionary Statements | P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | 25 |
| Safety Phrases | S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | OO4200000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2926909090 |
|
~98% 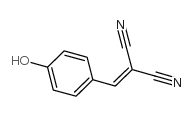
3785-90-8 |
| Literature: Gopalakrishna Panicker, Rajesh Krishnan; Krishnapillai, Sreekumar Tetrahedron Letters, 2014 , vol. 55, # 15 p. 2352 - 2354 |
|
~59% 
3785-90-8 |
| Literature: Sidhu, Anjali; Sharma; Rai, Mangat Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2010 , vol. 49, # 2 p. 247 - 250 |
|
~% 
3785-90-8 |
| Literature: Ertel,W.; Friedrich,K. Chemische Berichte, 1977 , vol. 110, p. 86 - 95 |
|
~% 
3785-90-8 |
| Literature: Ertel,W.; Friedrich,K. Chemische Berichte, 1977 , vol. 110, p. 86 - 95 |
|
~% 
3785-90-8
Detail
|
| Literature: Maggi, Raimondo; Ballini, Roberto; Sartori, Giovanni; Sartorio, Raffaella Tetrahedron Letters, 2004 , vol. 45, # 11 p. 2297 - 2299 |
| Precursor 6 | |
|---|---|
| DownStream 5 | |
| HS Code | 2926909090 |
|---|---|
| Summary | HS:2926909090 other nitrile-function compounds VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |


![[4-(2,2-dicyanoethenyl)phenyl] acetate structure](https://image.chemsrc.com/caspic/128/19310-87-3.png)

![[4-(2,2-dicyanoethenyl)phenyl] 4-heptanoyloxybenzoate structure](https://image.chemsrc.com/caspic/002/78016-54-3.png)