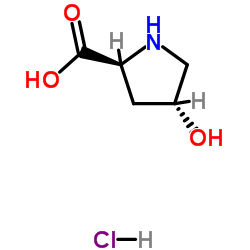
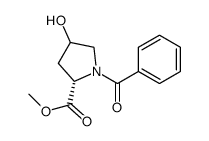
40216-83-9
| Name | (2S,4R)-Methyl 4-hydroxypyrrolidine-2-carboxylate hydrochloride |
|---|---|
| Synonyms |
Proline, 4-hydroxy-, methyl ester, hydrochloride (1:1)
Trans-4-hydroxy-L-proline methyl ester hydrochloride Methyl (4R)-4-hydroxy-L-prolinate hydrochloride (1:1) L-Proline, 4-hydroxy-, methyl ester, (4R)-, hydrochloride (1:1) Trans-4-Hydroxy-L-Proline Methyl Ester,Hydrochloride Salt (2S,4R)-4-Hydroxypyrrolidine-2-carboxylic Acid Methyl Ester Hydrochloride Methyl 4-hydroxyprolinate hydrochloride (1:1) L-4-Hydroxyproline methyl ester hydrochloride Methyl (2S,4R)-4-hydroxypyrrolidine-2-carboxylate Methyl (2S,4R)-4-Hydroxypyrrolidine-2-carboxylate Hydrochloride H-Hyp-OMe·HCl methyl 4-hydroxypyrrolidine-2-carboxylate hydrochloride MFCD00080855 H-Hyp-OMe.HCl trans-4-Hydroxy-L-proline methylester hydrochloride |
| Description | H-Hyp-OMe hydrochloride is a non-cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs). H-Hyp-OMe hydrochloride is also a alkyl chain-based PROTAC linker that can be used in the synthesis of PROTACs[1]< |
|---|---|
| Related Catalog | |
| Target |
Non-cleavable |
| In Vitro | ADCs are comprised of an antibody to which is attached an ADC cytotoxin through an ADC linker[1]. PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[2]. |
| References |
| Boiling Point | 247.2ºC at 760 mmHg |
|---|---|
| Molecular Formula | C6H12ClNO3 |
| Molecular Weight | 181.617 |
| Flash Point | 103.3ºC |
| Exact Mass | 181.050568 |
| PSA | 58.56000 |
| LogP | 0.01300 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
|
~88% 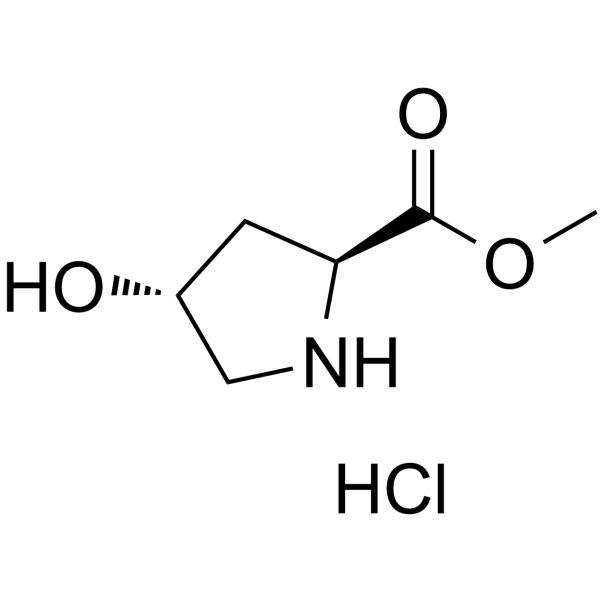
40216-83-9 |
| Literature: Shionogi Seiyaku Kabushiki Kaisha Patent: US5317016 A1, 1994 ; |
|
~98% 
40216-83-9 |
| Literature: INTERMUNE, INC.; BUCKMAN, Brad; NICHOLAS, John, B.; SEREBRYANY, Vladimir; SEIWERT, Scott, D. Patent: WO2012/37259 A1, 2012 ; Location in patent: Page/Page column 74 ; WO 2012/037259 A1 |
|
~93% 
40216-83-9 |
| Literature: ONO PHARMACEUTICAL CO., LTD; IMAGAWA, Akira; KONDO, Takashi; NISHIYAMA, Taihei; COURTNEY, Steve; YARNOLD, Chris; ICHIHARA, Osamu; FLANAGAN, Stuart Patent: WO2013/174937 A1, 2013 ; Location in patent: Page/Page column 74 ; |
|
~92% 
40216-83-9 |
| Literature: Cho, Han-Won; Oh, Chang-Hyun; Lee, Ju-Shin; Lee, Soo-Chul; Choi, Jung-Hoon; Cho, Jung-Hyuck Archiv der Pharmazie, 2003 , vol. 336, # 11 p. 495 - 503 |
|
~80% 
40216-83-9 |
| Literature: Papaioannou, Dionissios; Stavropoulos, George; Karagiannis, Kostas; Francis, George W.; Brekke, Trond; Aksnes, Dagfinn W. Acta Chemica Scandinavica, 1990 , vol. 44, # 3 p. 243 - 251 |
|
~99% 
40216-83-9 |
| Literature: Westwood, Nigel B.; Walker, Richard T. Tetrahedron, 1998 , vol. 54, # 44 p. 13391 - 13404 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |