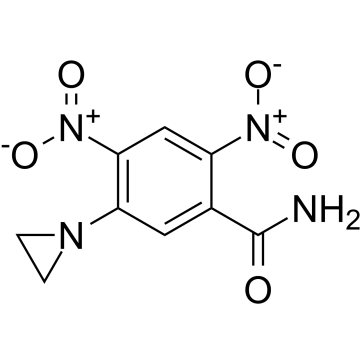
21919-05-1
| Name | 5-(aziridin-1-yl)-2,4-dinitrobenzamide |
|---|---|
| Synonyms |
5-(1-Aziridinyl)-2,4-dinitrobenzamide
2,4-Dinitro-5-ethyleneiminobenzamide 2,4-Dinitroethyleneiminobenzamide 5-(aziridyn-1-yl)-2,4-dinitrobenzamide 5-Aziridinyl-2,4-dinitrobenzamide CB-1954 5-(Aziridin-1-yl)-2,4-dinitrobenzamide Tretazicar 1 (5 carbamoyl 2,4 dinitrophenyl)aziridine UNII-7865D5D01M 5-aziridino-2,4-dinitrobenzamide |
| Description | Tretazicar (CB 1954), an antitumor prodrug, is highly selective against the Walker 256 rat tumour line. Tretazicar is enzymatically activated to generate a bifunctional agent, which can form DNA-DNA interstrand cross-links. Tretazicar in rat cells involves the reduction of its 4-nitro group to a 4-hydroxylamine by the enzyme NAD(P)H:quinone oxidoreductase 1 (NQO1)[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Tretazicar (CB 1954) (0.1-1000 μM; 3 days) has sensitivity for retrovirally transduced AB22 (AB22-nr) cells with an IC50 of 3 μM[3]. DNA cross-link formation in affected cells is a result of the bioactivation of the drug by the enzyme DT diaphorase (NAD(P)H dehydro-genase (quinone)) in the Walker cells which reduces the 4-nitro group of Tretazicar. The product of this reaction is a difunctional alkylating agent, 5-aziridin-1-yl-4-hydroxylamino-2-nitrobenzamide[4]. |
| In Vivo | Tretazicar (CB 1954) (80 mg/kg; i.p. on days 2 and 9) results in a significant increase in survival[3]. Animal Model: Female BALB/c mice (AB22-nr, SKOV3 human ovarian tumour xenograft)[3] Dosage: 80 mg/kg Administration: i.p. on days 2 and 9 Result: The median survival of the AB22-nr was 49 days. Resulted in a significant increase in survival. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 427.2±45.0 °C at 760 mmHg |
| Melting Point | 173 °C |
| Molecular Formula | C9H8N4O5 |
| Molecular Weight | 252.184 |
| Flash Point | 212.2±28.7 °C |
| Exact Mass | 252.049469 |
| PSA | 137.74000 |
| LogP | 1.28 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.715 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |