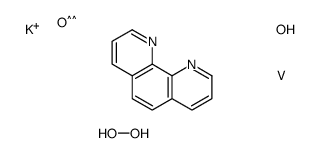
42494-73-5
| Name | potassium,hydrogen peroxide,oxovanadium,1,10-phenanthroline |
|---|
| Description | bpV(phen) is a potent protein tyrosine phosphatase (PTP) and PTEN inhibitor with IC50s of 38 nM, 343 nM and 920 nM for PTEN, PTP-β and PTP-1B. bpV(phen) is an insulin-mimetic agent following insulin-receptor tyrosine kinase hyperphosphorylation and activation. bpV(phen) activates HIV-1 transcription and replication via NF-κB-dependent and independent mechanisms. bpV(phen) inhibits proliferation of the protozoan parasite Leishmania in vitro. bpV(phen) strongly induces the secretion of a large number of chemokines and pro-inflammatory cytokines, and it activates a Th1-type pathway (IL-12, IFNγ). bpV(phen) can also induce cell apoptosis, and has anti-angiogenic and anti-tumor activity[1][2][3][4][5]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 38 nM (PTEN), 343 nM (PTP-β) and 920 nM (PTP-1B)[3] Parasite Leishmania[2] HIV-1[2] Apoptosis[1] |
| In Vitro | bpV(phen) (5 μM; 24.5 hours; H9c2 cells) treatment causes a further decrease of cell viability in H/R-injured H9c2 cells[1]. bpV(phen) (5 μM; 24.5 hours; H9c2 cells) treatment increases the apoptosis of H/R-injured H9c2 cells[1]. bpV(phen) (5 μM; 24.5 hours; H9c2 cells) treatment significantly promotes the accumulation of cytoplasmic Cytochrome C in H/R-injured H9c2 cells[1]. After stimulation of bpV(phen), PTEN-induced putative kinase protein 1 (PINK1)/Parkin-mediated mitophagy is inhibited[1]. Cell Viability Assay[1] Cell Line: Hypoxia/reoxygenation (H/R)-injured H9c2 cells Concentration: 5 μM Incubation Time: 24.5 hours (hypoxia for 24 h; reoxygenation for 30 minutes) Result: Caused a further decrease of cell viability. Apoptosis Analysis[1] Cell Line: Hypoxia/reoxygenation (H/R)-injured H9c2 cells Concentration: 5 μM Incubation Time: 24.5 hours (hypoxia for 24 h; reoxygenation for 30 minutes) Result: Increased the apoptosis of H/R-injured H9c2 cells. Western Blot Analysis[1] Cell Line: Hypoxia/reoxygenation (H/R)-injured H9c2 cells Concentration: 5 μM Incubation Time: 24.5 hours (hypoxia for 24 h; reoxygenation for 30 minutes) Result: Showed an increased release of Cytochrome C. |
| In Vivo | bpV(phen) (5 mg/kg; intraperitoneal injection; daily; for 38 days; male BALB/c nude (nu/nu) athymic mice) treatment causes a significant reduction in average tumor volume[1]. Animal Model: Male BALB/c nude (nu/nu) athymic mice (6-7 weeks old) injected with PC-3 cells[2] Dosage: 5 mg/kg Administration: Intraperitoneal injection; daily; for 38 days Result: Caused a significant reduction in average tumor volume. |
| References |
| Molecular Formula | C12H8KN2O5V |
|---|---|
| Molecular Weight | 354.27400 |
| Exact Mass | 353.98200 |
| PSA | 106.70000 |
| LogP | 2.69900 |
| Hazard Codes | Xi |
|---|