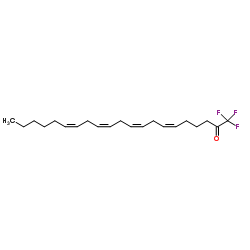
149301-79-1
| Name | aacocf3 |
|---|---|
| Synonyms |
Arachidonic acid trifluoromethyl ketone
AACOCF3 Arachidonyl trifluoromethyl ketone UNII:00XIW1CR0F Arachidonic acid trifluoromethylketone MFCD00236427 (6Z,9Z,12Z,15Z)-1,1,1-Trifluoro-6,9,12,15-henicosatetraen-2-one Arach-CF3 Arachidonyltrifluoromethane (6Z,9Z,12Z,15Z)-1,1,1-Trifluorohenicosa-6,9,12,15-tetraen-2-one |
| Description | AACOCF3 (Arachidonyl trifluoromethyl ketone) is a cell-permeant trifluoromethyl ketone analog of arachidonic acid. AACOCF3 is a potent and selective slow binding inhibitor of the 85-kDa cytosolic phospholipase A2 (cPLA2). AACOCF3 blocks production of arachidonate and 12-hydroxyeicosatetraenoic acid by calcium ionophore-challenged platelets. AACOCF3 inhibits glucose-induced insulin secretion from isolated rat islets. AACOCF3 has the potential for the research of cardiovascular disease[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | AACOCF3 inhibits the release of arachidonic acid from calcium ionophore-challenged U937 cells (IC50= 8 μM, 2 x 106 cells ml-1) and from platelets (IC50= 2 μM, 4 x 107 cells ml-1)[1]. AACOCF3 (10 μM) suppresses phosphate-induced calcification and osteogenic/chondrogenic signaling in HAoSMCs. AACOCF3 significantly inhibits both basal and Pi-induced release of arachidonic acid, the product of PLA2 activity[2]. |
| In Vivo | AACOCF3 (10 mg/kg; gavage; 5 days a week; ApoE–/– mice (6-week-old males) were fed a high-cholesterol diet) significantly reduces type III collagen plaque expression but had no significant influence on total collagen accumulation[3]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 421.7±45.0 °C at 760 mmHg |
| Molecular Formula | C21H31F3O |
| Molecular Weight | 356.465 |
| Flash Point | 288.7±20.2 °C |
| Exact Mass | 356.232697 |
| PSA | 17.07000 |
| LogP | 7.78 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.463 |
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |