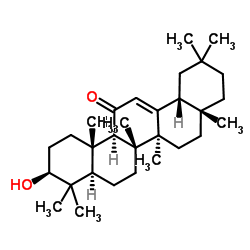
38242-02-3
| Name | (4aR,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-hydroxy-2,2,4a,6a,6b,9,9,12a-octamethyl-1,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,14b-octadecahydropicen-13(2H)-one |
|---|---|
| Synonyms |
β-Amyrenonol
b-Amyrenonol (3β)-3-Hydroxyolean-12-en-11-one 11-oxo-β-amyrin |
| Description | β-Amyrenonol (11-Oxo-β-amyrin), an oleanolic-type triterpenoid in licorice roots, is a precursor of Glycyrrhetinic acid. β-Amyrenonol has anti-proliferative and anti-inflammatory activities, and β-Amyrenonol could function as the skeleton for the synthesis of many triterpenoids[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | β-Amyrenonol (11-Oxo-β-amyrin) inhibits cell growth of HL60 cells with an IC50 value of 26.3 µM[1]. β-Amyrenonol (11-Oxo-β-amyrin) (100 μM) significantly reduces lipopolysaccharide-induced TNFα release in THP-1 cells[1]. CYP88D6 is characterized by in vitro enzymatic activity assays and shown to catalyze the sequential two-step oxidation of β-amyrin at C-11 to produce β-Amyrenonol (11-Oxo-β-amyrin). CYP88D6 coexpressed with β-amyrin synthase in yeast also catalyzed in vivo oxidation of β-amyrin to β-Amyrenonol[3]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 524.0±49.0 °C at 760 mmHg |
| Molecular Formula | C30H48O2 |
| Molecular Weight | 440.701 |
| Flash Point | 221.3±22.4 °C |
| Exact Mass | 440.365417 |
| PSA | 37.30000 |
| LogP | 8.61 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.545 |
| Hazard Codes | Xi |
|---|