578-86-9
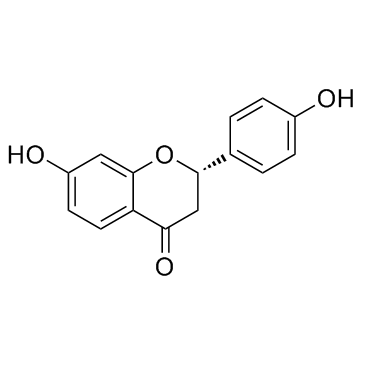
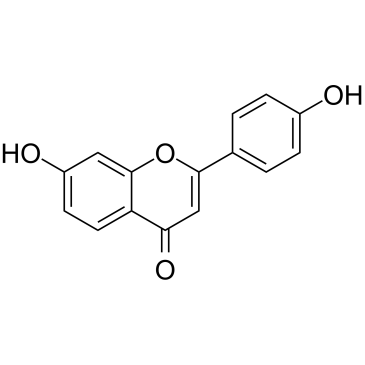
| Name | liquiritigenin |
|---|---|
| Synonyms |
(2S)-7-hydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one
(2S)-7-Hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one Liquiritigenin (2S)-7-Hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one |
| Description | Liquiritigenin, a flavanone isolated from Glycyrrhiza uralensis, is a highly selective estrogen receptor β (ERβ) agonist with an EC50 of 36.5 nM for activation of the ERE tk-Luc. |
|---|---|
| Related Catalog | |
| Target |
EC50: 36.5 nM (activation of the ERE tk-Luc)[1] |
| In Vitro | Liquiritigenin produces a dose-response activation of ERE tk-Luc in the U2OS cells transfected with ERβ, but not ERα. Liquiritigenin produces a dose-dependent activation and a time-dependent increase of the CECR6, NKG2E and NKD with ERβ but not with ERα. The ERβ-selectivity of liquiritigenin is due to the selective recruitment of the coactivator steroid receptor coactivator-2 to target genes. Liquiritigenin exhibits similar binding affinities for ERα and ERβ, and causes the recruitment of SRC-2 to target genes selectively in ERβ cells[1]. Pretreatment of MC3T3-E1 cells with liquiritigenin prevents the MG-induced cell death and production of protein adduct, intracellular reactive oxygen species, mitochondrial superoxide, cardiolipin peroxidation, and TNF-α in osteoblastic MC3T3-E1 cells[2]. |
| In Vivo | In a mouse xenograph model, liquiritigenin does not stimulate uterine size or tumorigenesis of MCF-7 breast cancer cells[1]. Treatment with liquiritigenin significantly reduces the concentrations of pro-inflammatory cytokines including interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α in serum and hippocampus[3]. |
| Kinase Assay | The relative binding affinity of liquiritigenin to pure full-length ERα and ERβ is determined using ERα and ERβ competitor assay kits. Fluorescence polarization of the fluorophore-tagged estrogen bound to ERα and ERβ in the presence of increasing amounts of competitor ligand or extract is determined using the GENios Pro microplate reader with fluorescein excitation (485 nM) and emission (530 nM) filters[1]. |
| Animal Admin | Mice: MCF-7 (250,000) cells are grafted under the kidney capsule of nude mice. Five mice per group are treated with a continuous infusion using osmotic pumps containing vehicle, E2 (0.4 mg) or liquiritigenin (2 mg) that infused 2.5 μL/h for 1 month. After one month of treatment, the tumors and uteri are removed and analyzed[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 529.5±50.0 °C at 760 mmHg |
| Melting Point | 206-208ºC |
| Molecular Formula | C15H12O4 |
| Molecular Weight | 256.253 |
| Flash Point | 206.9±23.6 °C |
| Exact Mass | 256.073547 |
| PSA | 66.76000 |
| LogP | 2.76 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.662 |
| Hazard Codes | Xi |
|---|---|
| HS Code | 29329990 |