890-38-0
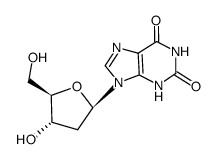
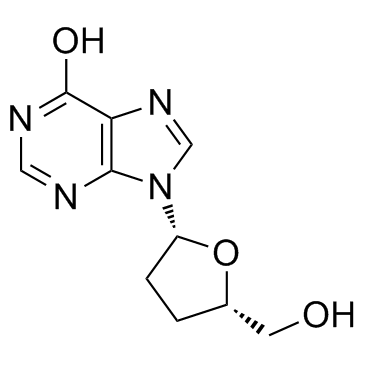
| Name | 2ʼ-deoxyinosine |
|---|---|
| Synonyms |
Inosine,2'-deoxy
EINECS 212-964-1 2'-Deoxyinosine 9-(2-deoxy-b-D-erythro-pentofuranosyl)-Hypoxanthine 9-(2-deoxy-β-D-erythro-pentofuranosyl)-Hypoxanthine 9-(2-Deoxy-b-D-erythro-pentofuranosyl)-1,9-dihydro-6H-purin-6-one 9-((2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-9H-purin-6-ol Deoxyinosine (-)-2'-Deoxyinosine d-Ino 2'-DEOXYGUANOSINE-5'-TRIPHOSPHATE TRISODIUM SALT,DGTP MFCD00005762 |
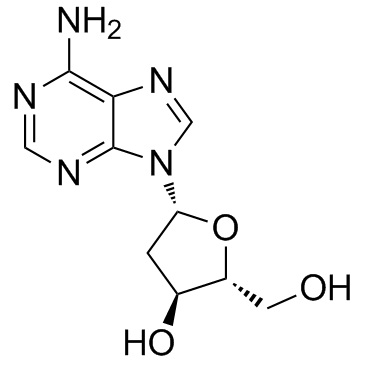
| Description | 2’-deoxyadenosine inhibits the growth of human colon-carcinoma cell lines and is found to be associated with purine nucleoside phosphorylase (PNP) deficiency. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
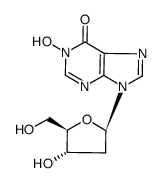
| In Vitro | In the absence of deoxycoformycin, 2’-deoxyadenosine is primarily deaminated to 2’-deoxyinosine and then converted into hypoxanthine. In the presence of the inhibitor, the deoxynucleoside, in addition to a phosphorylation process, undergoes phosphorolytic cleavage giving rise to adenine. The conversion of 2’-deoxyadenosine to adenine might represent a protective device, emerging when the activity of adenosine deaminase is reduced or inhibited. There is much evidence to indicate that the enzyme catalyzing this processs may be distinct from methylthioadenosine phosphorylase and S-adenosyl homocysteine hydrolase, which are the enzymes reported to be responsible for the formation of adenine from 28-deoxyadenosine in mammals[1]. |
| Cell Assay | Various amounts of cells, ranging from 1,000 to 900,000, suspended in 3 mL of standard medium, are plated in 35-mm dishes and incubated in the absence (control) and in the presence of both 1 μM dCF and 0.1 mM dAdo, as indicated in each experiment. dCf was added to the standard medium 30 min before 2’-deoxyadenosine (dAdo). Furthermore, an incubation is performed in which 0.1 mM 2’-deoxyadenosine alone is added to the standard medium. After 4 days of incubation, the standard medium is withdrawn, then 0.5 mL of 0.025% trypsin containing 0.02% EDTA is added and kept for few minutes at 37°C. The cells are collected, diluted in an appropriate volume of standard medium, and counted[1]. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 620.6±65.0 °C at 760 mmHg |
| Melting Point | 250 °C |
| Molecular Formula | C10H12N4O4 |
| Molecular Weight | 252.227 |
| Flash Point | 329.1±34.3 °C |
| Exact Mass | 252.085861 |
| PSA | 113.26000 |
| LogP | -1.43 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.837 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn,Xi |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2934999090 |
|
~92% 
890-38-0 |
| Literature: Journal of Organic Chemistry, , vol. 60, # 19 p. 6129 - 6134 |
|
~15% 
890-38-0 |
| Literature: Tetrahedron Letters, , vol. 39, # 40 p. 7397 - 7400 |
|
~% 
890-38-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 44, # 2 p. 288 - 295 |
|
~% 
890-38-0 |
| Literature: Chemical Research in Toxicology, , vol. 23, # 1 p. 48 - 54 |
| Precursor 3 | |
|---|---|
| DownStream 10 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


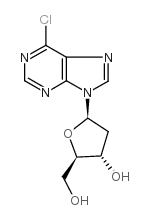
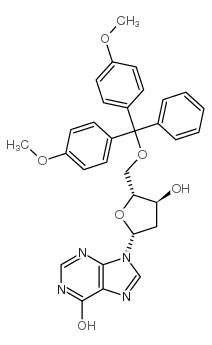
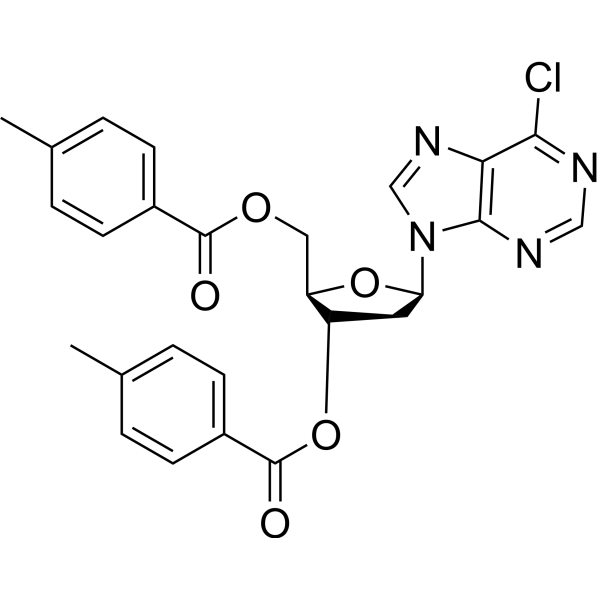
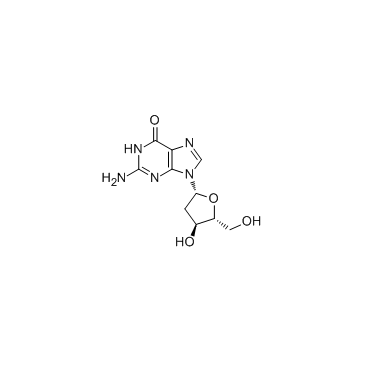
![9-[(2R,5S)-5-[[tert-butyl(dimethyl)silyl]oxymethyl]oxolan-2-yl]-3H-purin-6-one structure](https://image.chemsrc.com/caspic/418/177779-56-5.png)