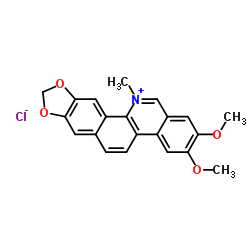
13063-04-2
| Name | 2,3-Dimethoxy-12-methyl-[1,3]dioxolo[4',5':4,5]benzo[1,2-c]phenanthridin-12-ium chloride |
|---|---|
| Synonyms |
2,3-Dimethoxy-12-methyl-[1,3]benzodioxolo[5,6-c]phenanthridinium chloride
[1,3]Benzodioxolo[5,6-c]phenanthridinium, 2,3-dimethoxy-12-methyl-, chloride (1:1) 2,3-Dimethoxy-12-methyl[1,3]benzodioxolo[5,6-c]phenanthridin-12-ium chloride Nitidine chloride 2,3-dimethoxy-12-methyl-[1,3]benzodioxolo[5,6-c]phenanthridin-12-ium,chloride |
| Description | Nitidine chloride, a potential anti-malarial lead compound derived from Zanthoxylum nitidum (Roxb) DC, exerts potent anticancer activity through diverse pathways, including inducing apoptosis, inhibiting STAT3 signaling cascade, DNA topoisomerase 1 and 2A, ERK and c-Src/FAK associated signaling pathway. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kB pathway[1][2][3][4][5][6]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.35g/cm3 |
|---|---|
| Boiling Point | 619ºC at 760 mmHg |
| Melting Point | 281-282ºC |
| Molecular Formula | C21H18ClNO4 |
| Molecular Weight | 383.825 |
| Flash Point | 189.4ºC |
| Exact Mass | 383.092438 |
| PSA | 40.80000 |
| LogP | 3.71660 |
| Appearance | white to beige |
| Vapour Pressure | 3.01E-15mmHg at 25°C |
| Index of Refraction | 1.696 |
| Storage condition | ?20°C |
| Water Solubility | DMSO: soluble1mg/mL, clear (warmed) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Safety Phrases | 24/25 |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | DF4935500 |
| HS Code | 2933990090 |
| Precursor 6 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |




![[1,3]Benzodioxolo[5,6-c]phenanthridine,12,13-dihydro-2,3-dimethoxy-12-methyl structure](https://image.chemsrc.com/caspic/379/13063-06-4.png)
![7,8-Dihydronaphtho[2,3-d][1,3]dioxol-5(6H)-one structure](https://image.chemsrc.com/caspic/113/41303-45-1.png)
![6,6-dibromo-7,8-dihydronaphtho[2,3-d][1,3]dioxol-5(6H)-one structure](https://image.chemsrc.com/caspic/278/180411-09-0.png)
![2,3-dimethoxy-[1,3]benzodioxolo[5,6-c]phenanthridine structure](https://image.chemsrc.com/caspic/379/18034-03-2.png)