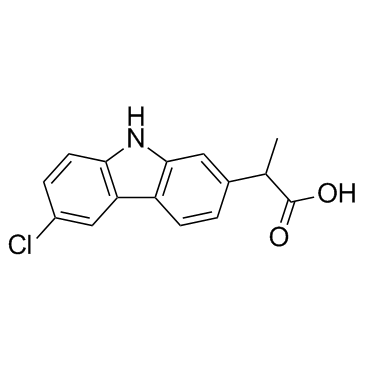
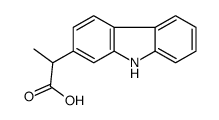
53716-49-7
| Name | carprofen |
|---|---|
| Synonyms |
UNII:FFL0D546HO
Imadyl Rimadyl MFCD00079028 2-(6-Chloro-9H-carbazol-2-yl)propanoic acid EINECS 258-712-4 Carprofen Ro-20-5720/000 6-Chloro-α-methyl-9H-carbazole-2-acetic Acid c5720 |
| Description | Carprofen is a nonsteroid anti-inflammatory agent, acts as a multi-target FAAH/COX inhibitor, with IC50s of 3.9 μM, 22.3 μM and 78.6 μM for COX-2, COX-1 and FAAH, respectively. |
|---|---|
| Related Catalog | |
| Target |
COX-2:3.9 μM (IC50) COX-1:22.3 μM (IC50) FAAH:78.6 μM (IC50) |
| In Vitro | Carprofen (Compound 1) is a nonsteroid anti-inflammatory agent, acts as a multi-target FAAH/COX inhibitor, with IC50s of 3.9 μM, 22.3 μM and 78.6 μM for COX-2, COX-1 and FAAH, respectively[1]. Carprofen (10 µg/mL) shows cytoprotective effects in CCL and CaCL cells and decreases apoptosis of both cells. Carprofen (10 µg/mL) exhibits nonsignificant increase in PGE2 concentration, compared with that of the respective CCL or CaCL controls[2]. |
| In Vivo | Carprofen (2.2 mg/kg, p.o.) significantly decreases PGE2 concentration in blood of dogs on days 3 and 10. Carprofen also decreases amounts of gastric PGE2 synthesis on day 3, but the inhibition is not obvious on day 10. In addition, Carprofen shows no activity against gastric PGE1 synthesis in dogs on day 3 and 10[3]. |
| Cell Assay | Cruciate ligament cells are used and incubated with DMEM supplemented with 10% FCS for 24 hours to synchronize cell cycles. The cell cultures are then preincubated without (control) or with a nonselective COX inhibitor (acetylsalicylic acid) or a preferential COX-2 inhibitor (Carprofen, meloxicam, or robenacoxib) to ssess whether NSAIDs prevented apoptosis when the cells are subsequently incubated with SNP. For all cell cultures except those designated as controls, 1 of 3 concentrations of 1 of the 4 NSAIDs (10, 100, or 200 µg of acetylsalicylic acid/mL; 0.1, 1, or 10 µg of Carprofend/mL; 0.1, 1, or 10 µg of meloxicame/mL; or 0.1, 1, or 10 µg of robenacoxibf/mL) is added to the culture media of each cell culture, and the cells are incubated for 2 hours[2]. |
| Animal Admin | Dogs[3] Each dog receives Carprofen (2.2 mg/kg, PO, q 12 h), deracoxib (2 mg/kg, PO, q 24 h), or etodolac (10 to 15 mg/kg, PO, q 24 h) for 10 days in a crossover design with a 30- to 60-day washout period between treatments. On days 0, 3, and 10 of each treatment period, blood is collected for evaluation of TXB2 and PGE2 concentrations. In addition, anesthesia is induced with propofol (4 mg/kg) and maintained with isoflurane. Synovial fluid is collected from both stifle joints by use of a standard arthrocentesis technique for evaluation of PGE2 concentrations. Gastroscopy is performed during each anesthetic episode, and 3 to 6 endoscopic biopsy specimens are collected from the gastric antrum for evaluation of PGE1 and PGE2 synthesis. On day 0 for each dog, a gastric biopsy specimen is placed into a Campylobacter-like organism test kit and evaluated for up to 24 hours for Helicobacter spp. Stained slides (H&E) of gastric biopsy specimens are also evaluated for the presence of underlying inflammation[3]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 509.1±35.0 °C at 760 mmHg |
| Melting Point | 186-188ºC |
| Molecular Formula | C15H12ClNO2 |
| Molecular Weight | 273.714 |
| Flash Point | 261.7±25.9 °C |
| Exact Mass | 273.055664 |
| PSA | 53.09000 |
| LogP | 4.03 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.732 |
| Storage condition | 0-6°C |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25 |
| Safety Phrases | S45 |
| RIDADR | UN 2811 |
| RTECS | FE3180000 |
| HS Code | 2933990090 |
| Precursor 7 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |