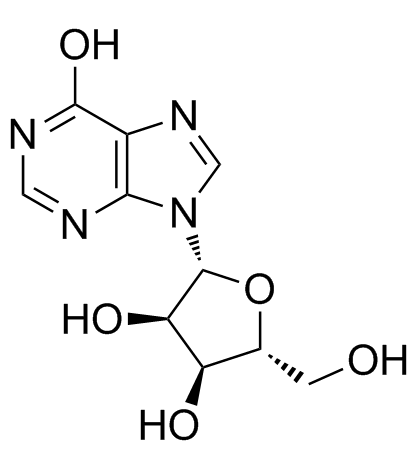
58-63-9
| Name | inosine |
|---|---|
| Synonyms |
6-Oxopurine riboside
HXR RIBOXINE nosine Ino EINECS 200-390-4 9-β-δ-Ribofuranosylhypoxanthine 1,9-dihydro-9-b-D-ribofuranosyl-6H-Purin-6-one 9-β-D-ribofuranosyl-Hypoxanthine 9-β-D-Ribofuranosylhypoxanthine Inosie 9-[(2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxyméthyl)tétrahydro-2-furanyl]-1,9-dihydro-6H-purin-6-one INO 495 hypoxanthine Oxiamin 9-b-D-Ribofuranosylhypoxanthine Atorel MFCD00066770 9β-D-Ribofuranosylhypoxanthine 6-oxy-purine riboside 9-β-D-ribofuranosyl-1,9-dihydro-6H-purin-6-one 9-b-D-ribofuranosyl-Hypoxanthine Inosine 9-[(2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-1,9-dihydro-6H-purin-6-one T56 BN DN FN HNJ IQ D- BT5OTJ CQ DQ E1Q &&β-D-Ribo Form Inosine (8CI,9CI) Selfer |
| Description | Inosine, an endogenous purine nucleoside, has immunomodulatory, neuroprotective, and analgesic properties. In vitro: Inosine has been shown to stimulate axonal growth in cell culture and promote corticospinal tract axons to sprout collateral branches after stroke, spinal cord injury and TBI in rodent models.[1] Inosine dose-dependently stimulates cAMP production mediated through the A2AR. Inosine dose-dependently induces A2AR-mediated ERK1/2 phosphorylation.[2] In vivo: The reference for Inosine is 1 or 10 mg/kg, i.p. Preventive treatment with inosine inhibits the development and progression of EAE in C57Bl/6 mice. neuroinflammation and demyelinating processes are blocked by inosine treatment. Additionally, inosine consistently inhibits IL-17 levels in peripheral lymphoid tissue, as well as IL-4 levels and A2AR up-regulation in the spinal cord, likely, through an ERK1-independent pathway. [3] inosine acting through adenosine receptors (ARs) exerts a wide range of anti-inflammatory and immunomodulatory effects in vivo. [2] |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 2.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 670.5±65.0 °C at 760 mmHg |
| Melting Point | 222-226 °C (dec.)(lit.) |
| Molecular Formula | C10H12N4O5 |
| Molecular Weight | 268.226 |
| Flash Point | 359.3±34.3 °C |
| Exact Mass | 268.080780 |
| PSA | 133.49000 |
| LogP | -1.91 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.879 |
| Water Solubility | 2.1 g/100 mL (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | NM7460000 |
| HS Code | 2934999090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


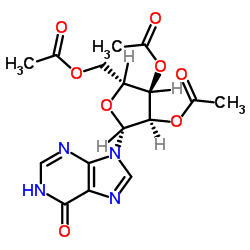
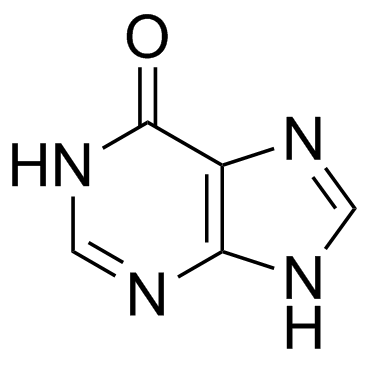

![9-[3,4-dihydroxy-5-(iodomethyl)oxolan-2-yl]-3H-purin-6-one structure](https://image.chemsrc.com/caspic/095/18945-36-3.png)