UNC 3230
Modify Date: 2024-01-09 10:43:53
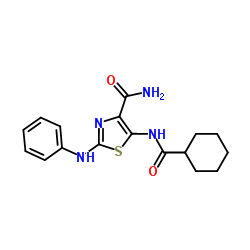
UNC 3230 structure
|
Common Name | UNC 3230 | ||
|---|---|---|---|---|
| CAS Number | 1031602-63-7 | Molecular Weight | 344.431 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C17H20N4O2S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of UNC 3230UNC3230 is a potent, selective and ATP-competitive phosphatidylinositol 4-phosphate 5 kinase type 1C (PIP5K1C) inhibitor with an IC50 of ~41 nM. UNC3230 also inhibits PIP4K2C and does not inhibit any of the other lipid kinases. UNC3230 has antinociceptive and anticancer effects[1]. |
| Name | 2-Anilino-5-[(cyclohexylcarbonyl)amino]-1,3-thiazole-4-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | UNC3230 is a potent, selective and ATP-competitive phosphatidylinositol 4-phosphate 5 kinase type 1C (PIP5K1C) inhibitor with an IC50 of ~41 nM. UNC3230 also inhibits PIP4K2C and does not inhibit any of the other lipid kinases. UNC3230 has antinociceptive and anticancer effects[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: ~41 nM (phosphatidylinositol 4-phosphate 5 kinase type 1C (PIP5K1C))[1] |
| In Vitro | Membrane PIP2 levels are significantly reduced by ~45% in dorsal root ganglia (DRG) neurons treated with 100 nM UNC3230 (~2-fold above the IC50) relative to vehicle controls. UNC3230 significantly reduces lysophosphatidic acid (LPA)-evoked calcium signaling in cultured DRG neurons relative to vehicle[1]. |
| In Vivo | UNC3230 (2 nmol) significantly increases noxious heat-evoked paw withdrawal latency for two hours after intrathecal injection in wild-type mice, indicating an antinociceptive effect[1]. UNC3230 (2 nmol; intrathecal injection) is administered then one hour later co-injected 1 nmol LPA with UNC3230 (2 nmol, intrathecal injection). UNC3230 significantly blunts thermal hyperalgesia and mechanical allodynia compared to vehicle[1]. UNC3230 (2 nmol; intrathecal injection) significantly blunts thermal hyperalgesia and mechanical allodynia in the complete Freund’s adjuvant (CFA)-inflamed hindpaw (relative to vehicle control) but does not affect thermal or mechanical sensitivity in the control (non-inflamed) hindpaw over a multiday time course[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C17H20N4O2S |
| Molecular Weight | 344.431 |
| Exact Mass | 344.130707 |
| LogP | 2.49 |
| Index of Refraction | 1.687 |
| Storage condition | -20°C |
| 2-Anilino-5-[(cyclohexylcarbonyl)amino]-1,3-thiazole-4-carboxamide |
| 4-Thiazolecarboxamide, 5-[(cyclohexylcarbonyl)amino]-2-(phenylamino)- |