Bisindolylmaleimide IV
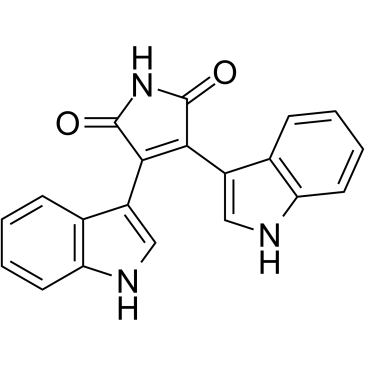
Bisindolylmaleimide IV structure
|
Common Name | Bisindolylmaleimide IV | ||
|---|---|---|---|---|
| CAS Number | 119139-23-0 | Molecular Weight | 327.336 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 690.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C20H13N3O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 371.2±31.5 °C | |
Use of Bisindolylmaleimide IVBisindolylmaleimide IV (Arcyriarubin A) is a potent protein kinase C (PKC) inhibitor, with IC50s ranging from 0.1 to 0.55 μM. Bisindolylmaleimide IV also inhibits PKA (IC50=3.1-11.8μM)[1]. Bisindolylmaleimide IV is a potent, selective inhibitor of human cytomegalovirus (HCMV) replication in cell culture with an IC50 of 0.2 μM[2]. |
| Name | Bisindolylmaleimide IV |
|---|---|
| Synonym | More Synonyms |
| Description | Bisindolylmaleimide IV (Arcyriarubin A) is a potent protein kinase C (PKC) inhibitor, with IC50s ranging from 0.1 to 0.55 μM. Bisindolylmaleimide IV also inhibits PKA (IC50=3.1-11.8μM)[1]. Bisindolylmaleimide IV is a potent, selective inhibitor of human cytomegalovirus (HCMV) replication in cell culture with an IC50 of 0.2 μM[2]. |
|---|---|
| Related Catalog | |
| Target |
PKC:0.1-0.55 μM (IC50) HCMV:0.2 μM (IC50) |
| In Vitro | Bisindolylmaleimide IV (Arcyriarubin A) inhibits PKC with an IC50 of 0.1 μM in 1 % DMSO, while the IC50 of 0.55 μM in 10 % DMSO[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 690.1±55.0 °C at 760 mmHg |
| Molecular Formula | C20H13N3O2 |
| Molecular Weight | 327.336 |
| Flash Point | 371.2±31.5 °C |
| Exact Mass | 327.100769 |
| PSA | 77.75000 |
| LogP | 3.21 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.811 |
| InChIKey | DQYBRTASHMYDJG-UHFFFAOYSA-N |
| SMILES | O=C1NC(=O)C(c2c[nH]c3ccccc23)=C1c1c[nH]c2ccccc12 |
| Storage condition | -20℃ |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 4 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Ca2+ influx through P2X1 receptors amplifies P2Y1 receptor-evoked Ca2+ signaling and ADP-evoked platelet aggregation.
Mol. Pharmacol. 86(3) , 243-51, (2014) Many cells express both P2X cation channels and P2Y G-protein-coupled receptors that are costimulated by nucleotides released during physiologic or pathophysiologic responses. For example, during hemo... |
|
|
High glucose-induced RhoA activation requires caveolae and PKCβ1-mediated ROS generation.
Am. J. Physiol. Renal Physiol. 302(1) , F159-72, (2012) Glomerular matrix accumulation is a hallmark of diabetic nephropathy. We previously showed that RhoA activation by high glucose in mesangial cells (MC) leads to matrix upregulation (Peng F, Wu D, Gao ... |
|
|
Entry of Newcastle Disease Virus into the host cell: role of acidic pH and endocytosis.
Biochim. Biophys. Acta 1838(1 Pt B) , 300-9, (2014) Most paramyxoviruses enter the cell by direct fusion of the viral envelope with the plasma membrane. Our previous studies have shown the colocalization of Newcastle Disease Virus (NDV) with the early ... |
| MFCD00236432 |
| 3,4-bis(1H-indol-3-yl)pyrrole-2,5-dione |
| 2,3-bis-(1h-indol-3-yl)-maleimide |
| 3,4-Bis(3-indolyl)maleimide |
| 3,4-Di(1H-indol-3-yl)-1H-pyrrole-2,5-dione |
| 1H-Pyrrole-2,5-dione, 3,4-di-1H-indol-3-yl- |
 CAS#:879-37-8
CAS#:879-37-8 CAS#:18372-22-0
CAS#:18372-22-0 CAS#:3005-27-4
CAS#:3005-27-4 CAS#:7553-56-2
CAS#:7553-56-2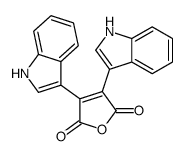 CAS#:115684-57-6
CAS#:115684-57-6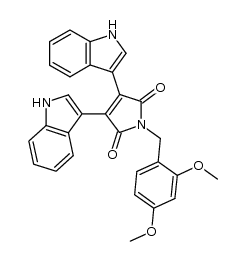 CAS#:342416-19-7
CAS#:342416-19-7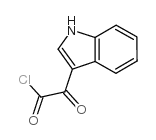 CAS#:22980-09-2
CAS#:22980-09-2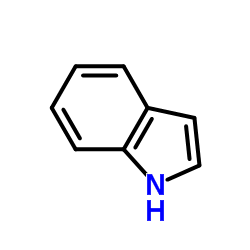 CAS#:120-72-9
CAS#:120-72-9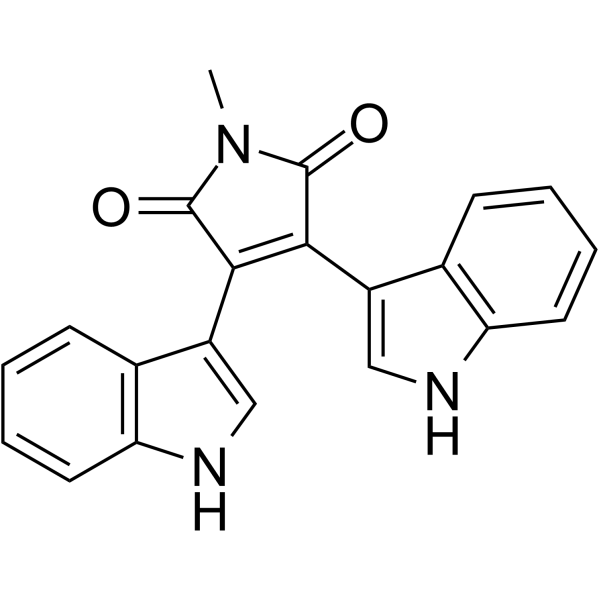 CAS#:113963-68-1
CAS#:113963-68-1 CAS#:1477-49-2
CAS#:1477-49-2 CAS#:133233-25-7
CAS#:133233-25-7 CAS#:133053-46-0
CAS#:133053-46-0 CAS#:118458-54-1
CAS#:118458-54-1
