Calphostin C
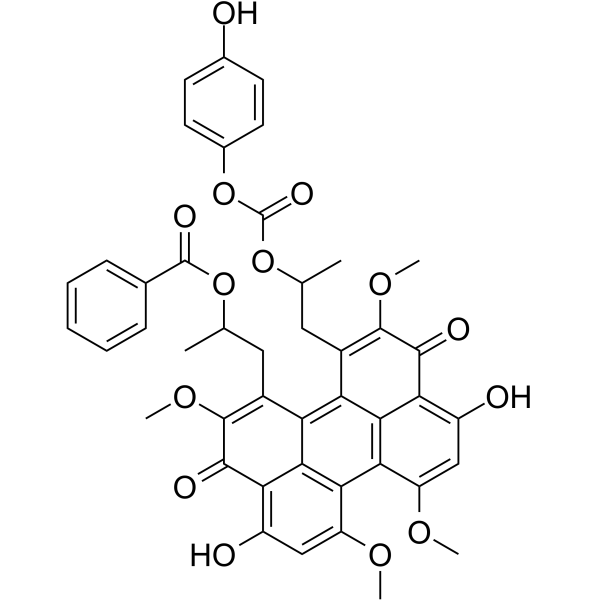
Calphostin C structure
|
Common Name | Calphostin C | ||
|---|---|---|---|---|
| CAS Number | 121263-19-2 | Molecular Weight | 790.764 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 1039.8±65.0 °C at 760 mmHg | |
| Molecular Formula | C44H38O14 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 317.0±27.8 °C | |
Use of Calphostin CCalphostin C is a potent and specific inhibitor of protein kinase C. Calphostin C is an antitumor antibiotic. Calphostin C has 1000 times more inhibitory to protein kinase C with an IC50 of 0.05 μM than other protein kinases. Calphostin C induces apoptosis in some tumor cell lines. Calphostin C has potent cytotoxic activity and antitumor activity[1]. |
| Name | Calphostin C from Cladosporium cladosporioides |
|---|---|
| Synonym | More Synonyms |
| Description | Calphostin C is a potent and specific inhibitor of protein kinase C. Calphostin C is an antitumor antibiotic. Calphostin C has 1000 times more inhibitory to protein kinase C with an IC50 of 0.05 μM than other protein kinases. Calphostin C induces apoptosis in some tumor cell lines. Calphostin C has potent cytotoxic activity and antitumor activity[1]. |
|---|---|
| Related Catalog | |
| Target |
protein kinase C[1] |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 1039.8±65.0 °C at 760 mmHg |
| Molecular Formula | C44H38O14 |
| Molecular Weight | 790.764 |
| Flash Point | 317.0±27.8 °C |
| Exact Mass | 790.226135 |
| PSA | 193.58000 |
| LogP | 9.15 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.705 |
| InChIKey | LSUTUUOITDQYNO-UHFFFAOYSA-N |
| SMILES | COc1c(O)c2c(=O)cc(OC)c3c4c(OC)cc(=O)c5c(O)c(OC)c(CC(C)OC(=O)c6ccccc6)c(c(c1CC(C)OC(=O)Oc1ccc(O)cc1)c23)c54 |
|
β-Adrenergic receptors activate exchange protein directly activated by cAMP (Epac), translocate Munc13-1, and enhance the Rab3A-RIM1α interaction to potentiate glutamate release at cerebrocortical nerve terminals.
J. Biol. Chem. 288(43) , 31370-85, (2013) The adenylyl cyclase activator forskolin facilitates synaptic transmission presynaptically via cAMP-dependent protein kinase (PKA). In addition, cAMP also increases glutamate release via PKA-independe... |
|
|
PPARγ regulates resistance vessel tone through a mechanism involving RGS5-mediated control of protein kinase C and BKCa channel activity.
Circ. Res. 111(11) , 1446-58, (2012) Activation of peroxisome proliferator-activated receptor-γ (PPARγ) by thiazolidinediones lowers blood pressure, whereas PPARγ mutations cause hypertension. Previous studies suggest these effects may b... |
|
|
Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BK(Ca) and Kir channels.
Am. J. Physiol. Heart Circ. Physiol. 302(6) , H1274-84, (2012) We hypothesized that chronic hyperglycemia has a detrimental effect on neurovascular coupling in the brain and that this may be linked to protein kinase C (PKC)-mediated phosphorylation. Therefore, in... |
| Carbonic acid, 2-[12-[2-(benzoyloxy)propyl]-4,9-dihydro-3,10-dihydroxy-2,6,7,11-tetramethoxy-4,9-dioxo-1-perylenyl]-1-methylethyl 4-hydroxyphenyl ester |
| Calphostin C |
| 1-[4,9-Dihydroxy-12-(2-{[(4-hydroxyphenoxy)carbonyl]oxy}propyl)-2,6,7,11-tetramethoxy-3,10-dioxo-3,10-dihydroperylen-1-yl]propan-2-yl benzoate |
| 1-[3,10-Dihydroxy-12-(2-{[(4-hydroxyphenoxy)carbonyl]oxy}propyl)-2,6,7,11-tetramethoxy-4,9-dioxo-4,9-dihydro-1-perylenyl]-2-propanyl benzoate |
| 1-[3,10-dihydroxy-12-[2-(4-hydroxyphenoxy)carbonyloxypropyl]-2,6,7,11-tetramethoxy-4,9-dioxoperylen-1-yl]propan-2-yl benzoate |
| Carbonic acid, 2-[12-[2-(benzoyloxy)propyl]-3,10-dihydro-4,9-dihydroxy-2,6,7,11-tetramethoxy-3,10-dioxo-1-perylenyl]-1-methylethyl 4-hydroxyphenyl ester |
| 1-[4,9-Dihydroxy-12-(2-{[(4-hydroxyphenoxy)carbonyl]oxy}propyl)-2,6,7,11-tetramethoxy-3,10-dioxo-3,10-dihydro-1-perylenyl]-2-propanyl benzoate |
| MFCD00133155 |

