LDN 193189 dihydrochloride
Modify Date: 2025-08-27 00:24:04
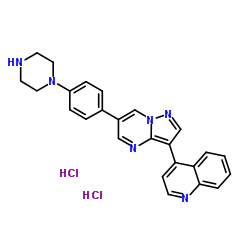
LDN 193189 dihydrochloride structure
|
Common Name | LDN 193189 dihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 1435934-00-1 | Molecular Weight | 479.40 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C25H24Cl2N6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of LDN 193189 dihydrochlorideLDN-193189 (dihydrochloride) is a potent selective BMP type I receptor (BMP I) inhibitor. LDN-193189 efficiently inhibits transcriptional activity of the BMP type I receptors ALK2 and ALK3 with IC50 values of 5 nM and 30 nM, respectively. LDN-193189 can be used for the research of bone morphogenetic protein signalling, such as fibrodysplasia ossificans progressiva[1][2][3]. |
| Name | 4-{6-[4-(1-Piperazinyl)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl}quinoline dihydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | LDN-193189 (dihydrochloride) is a potent selective BMP type I receptor (BMP I) inhibitor. LDN-193189 efficiently inhibits transcriptional activity of the BMP type I receptors ALK2 and ALK3 with IC50 values of 5 nM and 30 nM, respectively. LDN-193189 can be used for the research of bone morphogenetic protein signalling, such as fibrodysplasia ossificans progressiva[1][2][3]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C25H24Cl2N6 |
|---|---|
| Molecular Weight | 479.40 |
| Exact Mass | 478.143951 |
| InChIKey | CMQXLLAILGGLRV-UHFFFAOYSA-N |
| SMILES | Cl.Cl.c1ccc2c(-c3cnn4cc(-c5ccc(N6CCNCC6)cc5)cnc34)ccnc2c1 |
| Storage condition | -20℃ |
| Quinoline, 4-[6-[4-(1-piperazinyl)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-, hydrochloride (1:2) |
| 4-{6-[4-(1-Piperazinyl)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl}quinoline dihydrochloride |