N-Salicyloyltryptamine
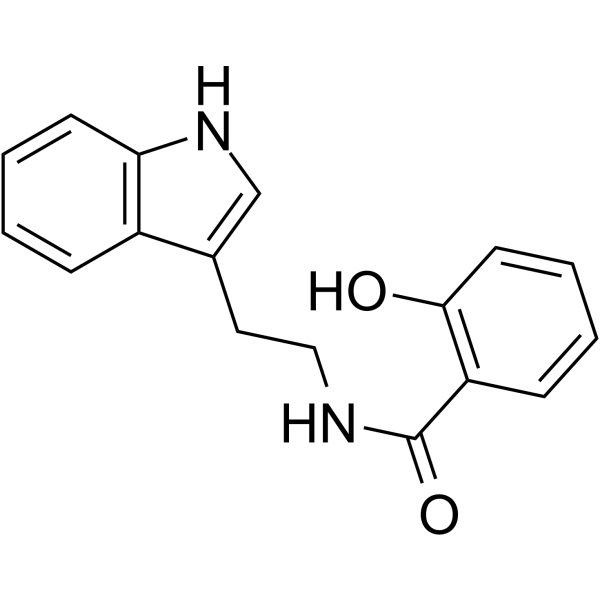
N-Salicyloyltryptamine structure
|
Common Name | N-Salicyloyltryptamine | ||
|---|---|---|---|---|
| CAS Number | 31384-98-2 | Molecular Weight | 280.32100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C17H16N2O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Purity | Quantity | Budget | Inquiry |
|---|
Use of N-SalicyloyltryptamineN-Salicyloyltryptamine acts on voltage-dependent Na+, Ca2+, and K+ ion channels inhibitor. N-Salicyloyltryptamine inhibits K+ currents with an IC50 value of 34.6 μM (Ito). N-Salicyloyltryptamine also exhibits anticonvulsant, anti-inflammatory, analgesic, and vasorelaxation effect[1]-[5]. |
| Name | 2-Hydroxy-N-[2-(1H-indol-3-yl)ethyl]benzamide |
|---|---|
| Synonym | More Synonyms |
| Description | N-Salicyloyltryptamine acts on voltage-dependent Na+, Ca2+, and K+ ion channels inhibitor. N-Salicyloyltryptamine inhibits K+ currents with an IC50 value of 34.6 μM (Ito). N-Salicyloyltryptamine also exhibits anticonvulsant, anti-inflammatory, analgesic, and vasorelaxation effect[1]-[5]. |
|---|---|
| Related Catalog | |
| Target |
L-type calcium channel potassium channel:34.6 μM (IC50) |
| In Vitro | N-Salicyloyltryptamine (1 ng/mL-1 μg/mL; 24 h) presents no cytotoxicity and causes no oxidative stress in RAW 264.7 cells at low concentration, but (50 and 100 μg/mL) inhibits cell viability with an IC50 value of 22.75 μg/mL[1]. N-Salicyloyltryptamine (1 μg/mL; 24 h) reverses some redox and inflammatory parameters induced by LPS without interfering in cell viability[1]. N-Salicyloyltryptamine (1 μg/mL; 24 h) inhibits LPS-induced TNF-α and IL-1β release, as well as CD40 and TNF-α protein up-regulation[1]. N-Salicyloyltryptamine (1 μg/mL; 24 h) inhibits phosphorylation of ERK 1/2 and IκBα and p65 nuclear translocation (NF-kB activation)[1]. N-Salicyloyltryptamine (17 μM) inhibits K+ current by 59.27% (Ito) and 73.18% (IKD), inhibits L-type Ca2+ currents by 54.9%, and shows few inhibition with high concentration (170 μM) on TTX-sensitive Na+ current by 22.1% in GH3 cells[2]. N-Salicyloyltryptamine (0.01 nM-100 µM) produces vasorelaxation through activation of the NO/sGC/cGMP pathway and reduction of calcium influx[3]. Cell Viability Assay[1] Cell Line: RAW 264.7 cell Concentration: 0.001, 0.05, 1, 50, 100 μg/mL Incubation Time: 24 hours Result: Resulted no effect on RAW 264.7 cell viability at 1 μg/mL; however, concentrations of 50 and 100 μg/mL significantly decreased both MTT reduction and SRB incorporation. RT-PCR[1] Cell Line: RAW 264.7 cell Concentration: 1 μg/mL Incubation Time: 24 hours Result: Reduced CD40, TNF-α, and RAGE immunocontent. Inhibited ERK1/2 and IκBα phosphorylation and nuclear translocation of p65. |
| In Vivo | N-Salicyloyltryptamine (100 mg/kg; i.p.; 60 min before stimulation challenge) significantly inhibits pentylenetetrazol (PTZ)-induced seizures and partially eliminates the extensor reflex of maximal electric-induced seizures test[4]. N-Salicyloyltryptamine (100 mg/kg, 200 mg/kg; i.p.; single dose) shows antinociceptive and nerve excitability effects[5]. Animal Model: Male Swiss mice (25-35 g)[4] Dosage: 50, 100, 200 mg/kg Administration: Intraperitoneal injection; single dose; 60 min before stimulation challenge Result: Reduced the incidence of clonic pentylenetetrazol (PTZ) seizures and mortality at 50 mg/kg, and decreased the incidence of tonic hindlimb extension (THE) produced by MES at 100, 200 mg/kg. Animal Model: Male Swiss mice (25-35 g)[5] Dosage: 100 mg/kg; 200 mg/kg Administration: Intraperitoneal injection; single dose Result: Reduced the acetic acid-induced licking response of the injected paw. |
| References |
| Molecular Formula | C17H16N2O2 |
|---|---|
| Molecular Weight | 280.32100 |
| Exact Mass | 280.12100 |
| PSA | 68.61000 |
| LogP | 3.42080 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Occurrence and behavior of the chiral anti-inflammatory drug naproxen in an aquatic environment.
Environ. Toxicol. Chem. 33(12) , 2671-8, (2014) The present study reports on the occurrence and chiral behavior of the anti-inflammatory drug (S)-naproxen (NAP)-(S)-2-(6-methoxynaphthalen-2-yl)propionic acid-in an aquatic environment under both fie... |
|
|
Nanoemulsions as novel oral carriers of stiripentol: insights into the protective effect and absorption enhancement.
Int. J. Nanomedicine 10 , 4937-46, (2015) Oral administration remains a significant challenge in regards to drugs with serious solubility and stability issues. This article aimed to investigate the suitability of nanoemulsions as oral carrier... |
|
|
Determination of natural and synthetic glucocorticoids in effluent of sewage treatment plants using ultrahigh performance liquid chromatography-tandem mass spectrometry.
Environ. Sci. Pollut. Res. Int. 22 , 14127-35, (2015) A sensitive and comprehensive analytical method for glucocorticoids (GCs) in water samples was developed and applied to effluent of sewage treatment plants (STPs). In the present study, totally 10 nat... |
| 2-hydroxy-N-(2-indol-3-yl-ethyl)-benzamide |
| N-Salicyloyltryptamine |
| N-Salicyloyltryptamin |