4-Nitrophenyl β-D-Cellobioside
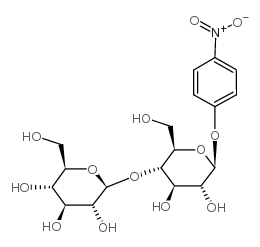
4-Nitrophenyl β-D-Cellobioside structure
|
Common Name | 4-Nitrophenyl β-D-Cellobioside | ||
|---|---|---|---|---|
| CAS Number | 3482-57-3 | Molecular Weight | 463.39000 | |
| Density | 1.7 g/cm3 | Boiling Point | 795.6ºC at 760 mmHg | |
| Molecular Formula | C18H25NO13 | Melting Point | 249-250ºC | |
| MSDS | Chinese USA | Flash Point | 435ºC | |
Use of 4-Nitrophenyl β-D-Cellobioside4-Nitrophenyl β-D-Cellobioside (p-Nitrophenyl β-D-cellobioside) is a cellotriose analog, a chromogenic substrate for the detection of cellulase activity. Exoglucanases, endoglucanases, and β-glucosidases hydrolyze 4-Nitrophenyl β-D-Cellobioside to yield p-nitrophenol (PNP)[1][2]. |
| Name | 4-Nitrophenyl β-D-cellobioside |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Nitrophenyl β-D-Cellobioside (p-Nitrophenyl β-D-cellobioside) is a cellotriose analog, a chromogenic substrate for the detection of cellulase activity. Exoglucanases, endoglucanases, and β-glucosidases hydrolyze 4-Nitrophenyl β-D-Cellobioside to yield p-nitrophenol (PNP)[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7 g/cm3 |
|---|---|
| Boiling Point | 795.6ºC at 760 mmHg |
| Melting Point | 249-250ºC |
| Molecular Formula | C18H25NO13 |
| Molecular Weight | 463.39000 |
| Flash Point | 435ºC |
| Exact Mass | 463.13300 |
| PSA | 224.35000 |
| Index of Refraction | 1.67 |
| Storage condition | 2-8°C |
| Water Solubility | methanol: water (2:3): 50 mg/mL, clear, faintly yellow |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29400090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
|
Mechanistic studies of active site mutants of Thermomonospora fusca endocellulase E2.
Biochemistry 38(30) , 9746-51, (1999) Endocellulase E2 from the thermophilic bacterium Thermomonospora fusca is a member of glycosyl-hydrolase family 6 and is active from pH 4 to 10. Enzymes in this family hydrolyze beta-1,4-glycosidic bo... |
|
|
Structural changes of cellobiohydrolase I (1,4-beta-D-glucan-cellobiohydrolase I, CBHI) and PNPC (p-nitrophenyl-beta-D-cellobioside) during the binding process.
Sci. China,. C, Life Sci. 51(5) , 459-69, (2008) Conformational changes to 1,4-beta-D-glucan cellobiohydrolase I (CBHI) in response to its binding with p-nitrophenyl beta-D-cellobioside (PNPC) were analyzed by second-derivative fluorescence spectrom... |
|
|
The competitive inhibition of Trichoderma reesei C30 cellobiohydrolase I by guanidine hydrochloride.
FEBS Lett. 270(1-2) , 143-6, (1990) The p-nitrophenylcellobiosidase (PNPCase) activity of Trichoderma reesei cellobiohydrolase I (CBH I) was competitively inhibited by concentrations of guanidine hydrochloride (Gdn HCl) that did not aff... |
| 4-NITROPHENYL-β-D-CELLOBIOSIDE |
| 4-Nitrophenyl β-D-Cellobioside |
| 4-NITROPHENYL-BETA-D-CELLOBIOSIDE |
| 4-Nitrophenyl beta-D-Cellobioside |
| MFCD00069845 |
 CAS#:69948-03-4
CAS#:69948-03-4 CAS#:2106-10-7
CAS#:2106-10-7 CAS#:2492-87-7
CAS#:2492-87-7 CAS#:5328-50-7
CAS#:5328-50-7 CAS#:133-99-3
CAS#:133-99-3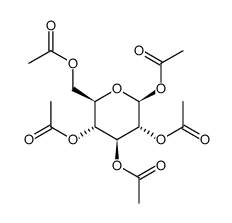 CAS#:83-87-4
CAS#:83-87-4 CAS#:5987-78-0
CAS#:5987-78-0 CAS#:100-02-7
CAS#:100-02-7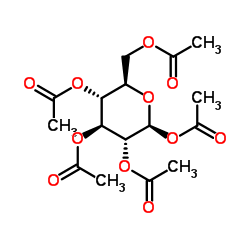 CAS#:604-69-3
CAS#:604-69-3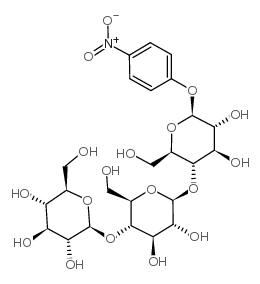 CAS#:106927-48-4
CAS#:106927-48-4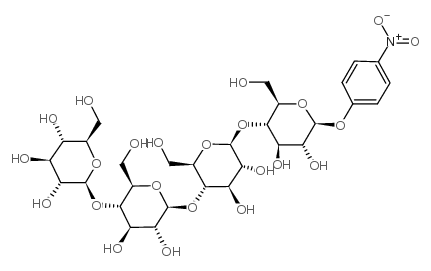 CAS#:129411-62-7
CAS#:129411-62-7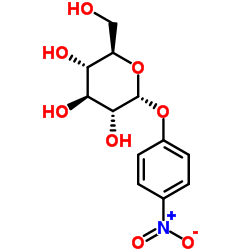 CAS#:3767-28-0
CAS#:3767-28-0 CAS#:492-62-6
CAS#:492-62-6 CAS#:619-08-9
CAS#:619-08-9 CAS#:6401-81-6
CAS#:6401-81-6 CAS#:42935-24-0
CAS#:42935-24-0
