Kinetin riboside
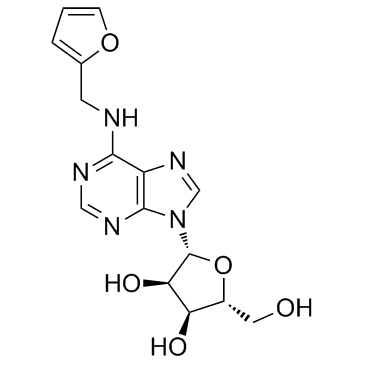
Kinetin riboside structure
|
Common Name | Kinetin riboside | ||
|---|---|---|---|---|
| CAS Number | 4338-47-0 | Molecular Weight | 347.326 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 683.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C15H17N5O5 | Melting Point | 152-154ºC | |
| MSDS | Chinese USA | Flash Point | 367.3±34.3 °C | |
Use of Kinetin ribosideKinetin riboside, a cytokinin analog, can induce apoptosis in cancer cells. It inhibits the proliferation of HCT-15 cells with an IC50 of 2.5 μM. |
| Name | kinetin riboside |
|---|---|
| Synonym | More Synonyms |
| Description | Kinetin riboside, a cytokinin analog, can induce apoptosis in cancer cells. It inhibits the proliferation of HCT-15 cells with an IC50 of 2.5 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 2.5 μM (HCT-15 cells)[1] |
| In Vitro | Kinetin riboside displays antiproliferative and apoptogenic activity against various human cancer cell lines. Kinetin riboside is able to inhibit the proliferation in HCT-15 human colon cancer cells in a dose-dependent manner (IC50=2.5 μM)[1]. Kinetin riboside induces apoptosis in HeLa and mouse melanoma B16F-10 cells. Kinetin riboside disrupts the mitochondrial membrane potential and induces the release of cytochrome c and activation of caspase-3. Bad are up-regulated while Bcl-2 is down-regulated under kinetin riboside exposure[2]. |
| In Vivo | Kinetin riboside significantly suppresses tumor growth. The most effective anti-melanoma response is elicited at 40 mg/kg[2]. |
| Cell Assay | HeLa and mouse melanoma B16F-10 cells are treated with 5, 10, 20 μM kinetin riboside for 48 h. 15 μL of MTT solution (5 mg/mL) is added to each well and cells are maintained for 4 h at 37°C. Hundred microlitres of solubilizing solution is then added. After an overnight incubation at room temperature, absorbance at 490 nm is measured[2]. |
| Animal Admin | Mice: Male C57BL/6 mice are injected B16 F-10 cells. After 5 days for tumor growth, kinetin riboside (10, 20, 40 mg/kg) is injected to tumor mass directly. Drug injection is performed once a 3 days for three times. After third injection of drug, mice are kept for 3 days with no injection and tumor mass is removed from each mouse and weighed[2]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 683.7±65.0 °C at 760 mmHg |
| Melting Point | 152-154ºC |
| Molecular Formula | C15H17N5O5 |
| Molecular Weight | 347.326 |
| Flash Point | 367.3±34.3 °C |
| Exact Mass | 347.122955 |
| PSA | 138.69000 |
| LogP | 0.20 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.798 |
| InChIKey | CAGLGYNQQSIUGX-SDBHATRESA-N |
| SMILES | OCC1OC(n2cnc3c(NCc4ccco4)ncnc32)C(O)C1O |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | AU7400200 |
| HS Code | 29349990 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
|
High-internal-phase-emulsion polymeric monolith coupled with liquid chromatography-electrospray tandem mass spectrometry for enrichment and sensitive detection of trace cytokinins in plant samples.
Anal. Bioanal. Chem 407 , 6071-9, (2015) High-internal-phase-emulsion polymers (polyHIPEs) show great promise as solid-phase-extraction (SPE) materials because of the tremendous porosity and highly interconnected framework afforded by the hi... |
|
|
Constrained NBMPR analogue synthesis, pharmacophore mapping and 3D-QSAR modeling of equilibrative nucleoside transporter 1 (ENT1) inhibitory activity.
Bioorg. Med. Chem. 16 , 3848-65, (2008) Conformationally constrained analogue synthesis was undertaken to aid in pharmacophore mapping and 3D-QSAR analysis of nitrobenzylmercaptopurine riboside (NBMPR) congeners as equilibriative nucleoside... |
|
|
Ribose-modified purine nucleosides as ribonucleotide reductase inhibitors. Synthesis, antitumor activity, and molecular modeling of N6-substituted 3'-C-methyladenosine derivatives.
J. Med. Chem. 51 , 4260-9, (2008) A series of cycloalkyl, bicycloalkyl, aryl, and heteroaryl N (6)-substituted derivatives of the antitumor agent 3'- C-methyladenosine (3'-Me-Ado), an inhibitor of the alpha Rnr1 subunit of mammalian r... |
| 9H-Purin-6-amine, N-(2-furanylmethyl)-9-pentofuranosyl- |
| N-(2-Furylmethyl)-9-pentofuranosyl-9H-purin-6-amine |
| furfuryladenosine |
| EINECS 224-389-3 |
| 6-Furfurylaminopurine riboside (N6-(2-Furanylmethyl)adenosine |
| Kinetin-9-riboside |
| MFCD00037987 |
| n-furfuryl-adenosin |
| Ribosylkinetin |
| N-Furfuryladenosine |
 CAS#:4753-68-8
CAS#:4753-68-8 CAS#:2004-06-0
CAS#:2004-06-0 CAS#:98-01-1
CAS#:98-01-1 CAS#:58-61-7
CAS#:58-61-7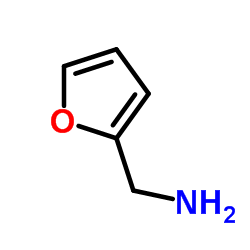 CAS#:617-89-0
CAS#:617-89-0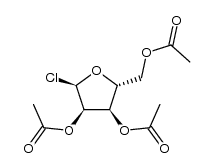 CAS#:105499-44-3
CAS#:105499-44-3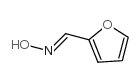 CAS#:1121-47-7
CAS#:1121-47-7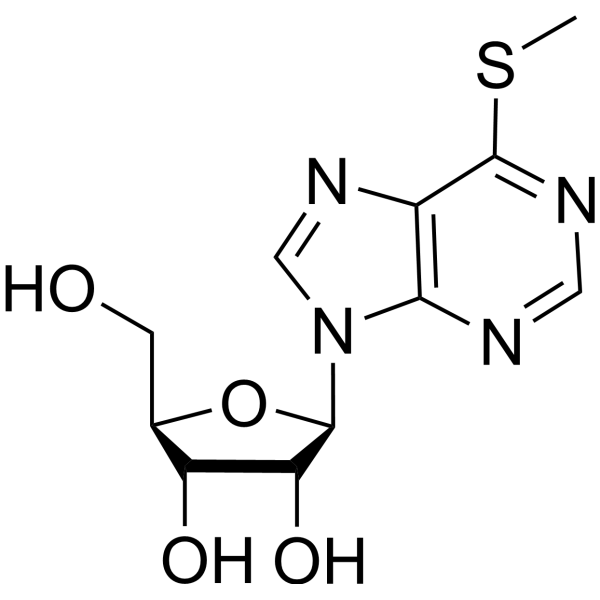 CAS#:342-69-8
CAS#:342-69-8![[5-(6-acetamidopurin-9-yl)-3,4-diacetyloxy-oxolan-2-yl]methyl acetate Structure](https://image.chemsrc.com/caspic/032/7387-58-8.png) CAS#:7387-58-8
CAS#:7387-58-8 CAS#:525-79-1
CAS#:525-79-1 CAS#:18646-11-2
CAS#:18646-11-2