Azetidine
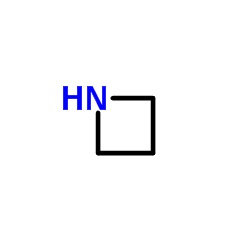
Azetidine structure
|
Common Name | Azetidine | ||
|---|---|---|---|---|
| CAS Number | 503-29-7 | Molecular Weight | 57.094 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 64.9±8.0 °C at 760 mmHg | |
| Molecular Formula | C3H7N | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | -23.6±16.5 °C | |
| Symbol |


GHS02, GHS05 |
Signal Word | Danger | |
| Name | azetidine |
|---|---|
| Synonym | More Synonyms |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 64.9±8.0 °C at 760 mmHg |
| Molecular Formula | C3H7N |
| Molecular Weight | 57.094 |
| Flash Point | -23.6±16.5 °C |
| Exact Mass | 57.057850 |
| PSA | 12.03000 |
| LogP | -0.20 |
| Vapour Pressure | 161.5±0.1 mmHg at 25°C |
| Index of Refraction | 1.426 |
| Storage condition | 2-8°C |
| Water Solubility | miscible |
| Symbol |


GHS02, GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H314 |
| Precautionary Statements | P210-P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US) |
| Hazard Codes | F: Flammable;C: Corrosive; |
| Risk Phrases | R11;R34 |
| Safety Phrases | S16-S26-S36/37/39-S45 |
| RIDADR | UN 2733 3/PG 2 |
| WGK Germany | 3 |
| Packaging Group | I |
| Hazard Class | 3.1 |
| HS Code | 29339990 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis from D-altrose of (5R,6R,7R,8S)-5,7-dihydroxy-8-hydroxymethylconidine and 2,4-dideoxy-2,4-imino-D-glucitol, azetidine analogues of swainsonine and 1,4-dideoxy-1,4-imino-D-mannitol.
Org. Lett. 14(16) , 4174-7, (2012) Ring closure of a 3,5-di-O-triflate derived from D-altrose with benzylamine allowed the formation of both monocyclic and bicyclic azetidine analogues of swainsonine. |
|
|
Identification of spirocyclic piperidine-azetidine inverse agonists of the ghrelin receptor.
Bioorg. Med. Chem. Lett. 22(13) , 4281-7, (2012) The discovery of spirocyclic piperidine-azetidine inverse agonists of the ghrelin receptor is described. The characterization and redressing of the issues associated with these compounds is detailed. ... |
|
|
Complex N-heterocycle synthesis via iron-catalyzed, direct C-H bond amination.
Science 340(6132) , 591-5, (2013) The manipulation of traditionally unreactive functional groups is of paramount importance in modern chemical synthesis. We have developed an iron-dipyrrinato catalyst that leverages the reactivity of ... |
| Azetidine |
| MFCD00005165 |
| Trimethylenimine |
| EINECS 207-963-8 |
| 1,3-Propylenimine |
| Azetidin |
| trimethyleneimine |
| Azacyclobutane |
| UNII-37S883XDWR |
 CAS#:78797-58-7
CAS#:78797-58-7 CAS#:6788-85-8
CAS#:6788-85-8 CAS#:7730-45-2
CAS#:7730-45-2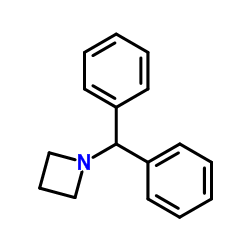 CAS#:107128-00-7
CAS#:107128-00-7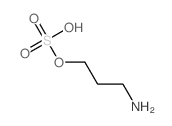 CAS#:1071-29-0
CAS#:1071-29-0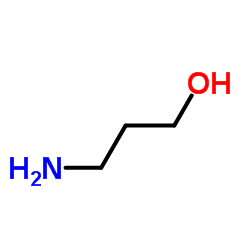 CAS#:156-87-6
CAS#:156-87-6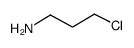 CAS#:14753-26-5
CAS#:14753-26-5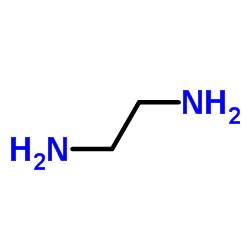 CAS#:107-15-3
CAS#:107-15-3 CAS#:18370-81-5
CAS#:18370-81-5 CAS#:530081-57-3
CAS#:530081-57-3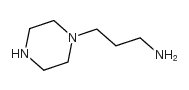 CAS#:34885-02-4
CAS#:34885-02-4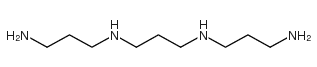 CAS#:4605-14-5
CAS#:4605-14-5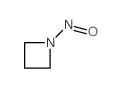 CAS#:15216-10-1
CAS#:15216-10-1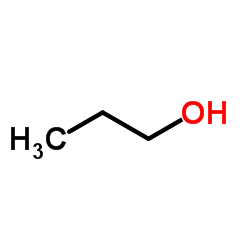 CAS#:71-23-8
CAS#:71-23-8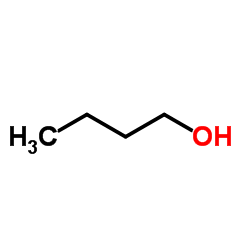 CAS#:71-36-3
CAS#:71-36-3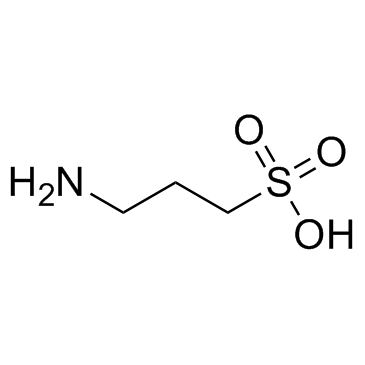 CAS#:3687-18-1
CAS#:3687-18-1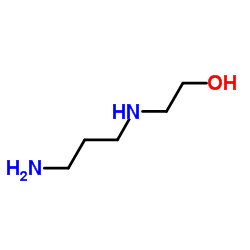 CAS#:4461-39-6
CAS#:4461-39-6 CAS#:632-22-4
CAS#:632-22-4
