Adrenaline

Adrenaline structure
|
Common Name | Adrenaline | ||
|---|---|---|---|---|
| CAS Number | 51-43-4 | Molecular Weight | 183.204 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 413.1±40.0 °C at 760 mmHg | |
| Molecular Formula | C9H13NO3 | Melting Point | 208-211ºC | |
| MSDS | Chinese USA | Flash Point | 207.9±17.9 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of AdrenalineL-Epinephrine is a hormone secreted by the medulla of the adrenal glands. L-Epinephrine is an α-adrenergic and β-adrenergic receptor agonist. |
| Name | (R)-adrenaline |
|---|---|
| Synonym | More Synonyms |
| Description | L-Epinephrine is a hormone secreted by the medulla of the adrenal glands. L-Epinephrine is an α-adrenergic and β-adrenergic receptor agonist. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vivo | A 25 microliter volume of a 1-% L-epinephrine borate solution applied on the cornea of one eye in 12 monkeys reduces blood flow through the iris and the ciliary body by 59% and 20%, respectively, compared to the untreated control eyes[1]. Epinephrine is a direct-acting sympathomimetic α-adrenergic and β-adrenergic agonist with cyclic adenosine monophosphate-mediated, complex, bidirectional pharmacologic effects on many target organs[2]. In young adult rats, endogenous release of epinephrine facilitates stable memory formation for temporally associated events. Epinephrine enhances memory in young adult rats, in part, by increasing blood glucose levels needed to modulate memory[3]. Epinephrine is the primary drug administered during cardiopulmonary resuscitation (CPR) to reverse cardiac arrest. Epinephrine increases arterial blood pressure and coronary perfusion during CPR via alpha-1-adrenoceptor agonist effects[4]. |
| Animal Admin | Rats: For the immunohistochemistry experiments, rats receive an immediate post-training subcutaneous injection of saline (0.9%), glucose (250 mg/kg), or epinephrine (0.1 mg/kg) and are then returned to the holding cage[3]. |
| References |
[4]. Callaway CW, et al. Epinephrine for cardiac arrest. Curr Opin Cardiol. 2013 Jan;28(1):36-42. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 413.1±40.0 °C at 760 mmHg |
| Melting Point | 208-211ºC |
| Molecular Formula | C9H13NO3 |
| Molecular Weight | 183.204 |
| Flash Point | 207.9±17.9 °C |
| Exact Mass | 183.089539 |
| PSA | 72.72000 |
| LogP | -0.63 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.608 |
Synonym:Epinephrine; Adrenal; 1-Adrenaline; Adrenaso Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 23/24/25 33 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Toxic by inhalation, in contact with skin and if swallowed. Danger of cumulative effects.Toxic. Potential Health Effects Eye: May cause eye irritation. No information regarding eye irritation and other potential effects was found. Skin: May cause skin irritation. No information regarding skin irritation and other potential effects was found. Ingestion: Causes gastrointestinal irritation with nausea, vomiting and diarrhea. Inhalation: May cause respiratory tract irritation. Overexposure may cause dizziness, tremors, restlessness, rapid heart beat, increased blood pressure, hallucinations, acidosis, kidney failure. Chronic: Not available. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Extinguishing Media: For small fires, use water spray, dry chemical, carbon dioxide or chemical foam. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Wash hands before eating. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Do not get on skin or in eyes. Do not ingest or inhale. Storage: Keep away from heat and flame. Store in a cool, dry, well-ventilated area away from incompatible substances. Keep containers tightly closed. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 51-43-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Color: white Odor: odorless pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: Not available. Autoignition Temperature: Not applicable. Flash Point: Not applicable. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Not available. Solubility in water: Not available. Specific Gravity/Density: Not available. Molecular Formula: C9H14ClNO3 Molecular Weight: 219.5559 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Incompatible materials. Incompatibilities with Other Materials: Strong oxidizers. Hazardous Decomposition Products: Hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 51-43-4: DO2625000 LD50/LC50: CAS# 51-43-4: Skin, rat: LD50 = 62 mg/kg. Carcinogenicity: 1,2-benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl] - Not listed by ACGIH, IARC, or NTP. Other: See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: ALKALOIDS, SOLID, NOS((-)-Adrenaline) Hazard Class: 6.1 UN Number: 1544 Packing Group: II IMO Shipping Name: ALKALOIDS, SOLID, NOS((-)-Adrenaline) Hazard Class: 6.1 UN Number: 1544 Packing Group: II RID/ADR Shipping Name: ALKALOIDS, SOLID, NOS((-)-Adrenaline) Hazard Class: 6.1 UN Number: 1544 Packing group: II USA RQ: CAS# 51-43-4: 1000 lb final RQ; 454 kg final RQ Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: T Risk Phrases: R 23/24/25 Toxic by inhalation, in contact with skin and if swallowed. R 33 Danger of cumulative effects. Safety Phrases: S 23 Do not inhale gas/fumes/vapour/spray. S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 51-43-4: 3 Canada CAS# 51-43-4 is listed on Canada's DSL List. CAS# 51-43-4 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 51-43-4 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 + H331-H310 |
| Precautionary Statements | P261-P280-P301 + P310-P302 + P350-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R23/24/25;R33;R36/37/38;R52/53 |
| Safety Phrases | S23-S26-S36/37/39-S45-S61 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | DO2625000 |
| Packaging Group | II |
| Hazard Class | 6.1 |
| HS Code | 2922509090 |
|
~52% 
Adrenaline CAS#:51-43-4 |
| Literature: Muller; Keil; Gerhard Tetrahedron, 1986 , vol. 42, # 5 p. 1529 - 1532 |
|
~% 
Adrenaline CAS#:51-43-4 |
| Literature: WOCKHARDT RESEARCH CENTRE; YADAV, RAMPRASAD; SHAIKH, ZAKIR GAFOOR; NASIR ALI, SHAFAKAT ALI; MERWADE, ARVIND; SIDDIQUI MOHAMMAD, JAWEED MUKARRAM Patent: WO2009/4593 A2, 2009 ; Location in patent: Page/Page column 7 ; |
|
~97% 
Adrenaline CAS#:51-43-4 |
| Literature: Singer, Robert A.; Carreira, Erick M. Tetrahedron Letters, 1997 , vol. 38, # 6 p. 927 - 930 |
|
~% 
Adrenaline CAS#:51-43-4 |
| Literature: Muller; Keil; Gerhard Tetrahedron, 1986 , vol. 42, # 5 p. 1529 - 1532 |
|
~% 
Adrenaline CAS#:51-43-4 |
| Literature: Muller; Keil; Gerhard Tetrahedron, 1986 , vol. 42, # 5 p. 1529 - 1532 |
|
~% 
Adrenaline CAS#:51-43-4 |
| Literature: Singer, Robert A.; Carreira, Erick M. Tetrahedron Letters, 1997 , vol. 38, # 6 p. 927 - 930 |
|
~% 
Adrenaline CAS#:51-43-4 |
| Literature: Singer, Robert A.; Carreira, Erick M. Tetrahedron Letters, 1997 , vol. 38, # 6 p. 927 - 930 |
|
~% 
Adrenaline CAS#:51-43-4 |
| Literature: Ges. f. Chem. Ind. Basel Patent: DE505460 ; Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 16, p. 2098 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Over-expression of Follistatin-like 3 attenuates fat accumulation and improves insulin sensitivity in mice.
Metab. Clin. Exp. 64(2) , 283-95, (2015) Follistatin-like 3 (fstl3), a natural inhibitor of members of the TGF-β family, increases during resistance training in human plasma. Fstl3 primarily binds myostatin and activin A, and thereby inhibit... |
|
|
Phospholipase C-related catalytically inactive protein (PRIP) regulates lipolysis in adipose tissue by modulating the phosphorylation of hormone-sensitive lipase.
PLoS ONE 9(6) , e100559, (2014) Phosphorylation of hormone-sensitive lipase (HSL) and perilipin by protein kinase A (PKA) promotes the hydrolysis of lipids in adipocytes. Although activation of lipolysis by PKA has been well studied... |
|
|
Endogenous brain pericytes are widely activated and contribute to mouse glioma microvasculature.
PLoS ONE 10(4) , e0123553, (2015) Glioblastoma multiforme (GBM) is the most common brain tumor in adults. It presents an extremely challenging clinical problem, and treatment very frequently fails due to the infiltrative growth, facil... |
| Epipen |
| L(-)-Epinephrine |
| 4-[(1R)-1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol |
| 4-[(1R)-1-Hydroxy-2-(methylamino)ethyl]benzol-1,2-diol |
| l-Epinephrine |
| (R)-Epinephrine |
| Lyodrin |
| 4-[(1R)-1-hydroxy-2-(méthylamino)éthyl]benzène-1,2-diol |
| Ana-Kit |
| Epifrin |
| (-)-3,4-dihydroxy-a-[(methylamino)methyl]-Benzyl alcohol |
| Corisol |
| L-Methylaminoethanolcatechol |
| Adrine |
| (-)-Adrenalin |
| (−)-Adrenalin |
| (−)-Epinephrine |
| adrenaline |
| R-(-)-Epinephrine |
| L-Adrenaline |
| (R)-(-)-adrenaline |
| (-)-adrenaline |
| 1,2-Benzenediol, 4-[(1R)-1-hydroxy-2-(methylamino)ethyl]- |
| L-Epinehphrine |
| MFCD00002204 |
| Bosmin |
| L-Epinephine |
| 4-[(1R)-1-Hydroxy-2-(methylamino)ethyl]-1,2-benzenediol |
| (-)-Epinephrine |
| (R)-adrenaline |
| (-)-3,4-Dihydroxy-a-[(methylamino)methyl]benzyl Alcohol |
| (S)-4-(1-hydroxy-2-(methylamino)ethyl)benzene-1,2-diol |
| 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- |
| Adrenan |
| EINECS 200-098-7 |
| (-)-R-Epinephrine |
| IOP |
| Levo-Methylaminoethanolcatechol |
| Adrenal |
| Suprel |
| (R)-(-)-Epinephrine |
| Levoreninum |
| Levophed |
| (-)-3,4-dihydroxy-α-[(methylamino)methyl]-Benzyl alcohol |
| Adrin |
| L-Adrenalin |
| Exadrin |
| Adrenalin |
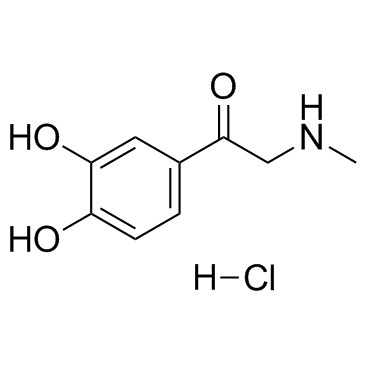

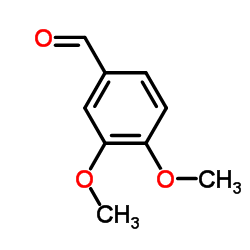
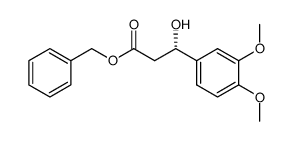
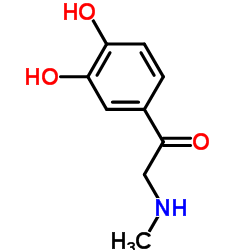
 CAS#:74-89-5
CAS#:74-89-5 CAS#:64-18-6
CAS#:64-18-6 CAS#:144-62-7
CAS#:144-62-7 CAS#:99-50-3
CAS#:99-50-3 CAS#:120-80-9
CAS#:120-80-9 CAS#:121-33-5
CAS#:121-33-5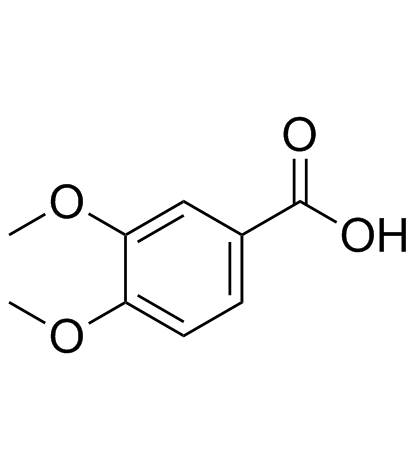 CAS#:93-07-2
CAS#:93-07-2 CAS#:75-50-3
CAS#:75-50-3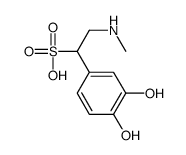 CAS#:26405-77-6
CAS#:26405-77-6