Kaempferol

Kaempferol structure
|
Common Name | Kaempferol | ||
|---|---|---|---|---|
| CAS Number | 520-18-3 | Molecular Weight | 286.236 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 582.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C15H10O6 | Melting Point | 276°C | |
| MSDS | Chinese USA | Flash Point | 226.1±23.6 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of KaempferolKaempferol inhibits estrogen receptor α expression in breast cancer cells and induces apoptosis in glioblastoma cells and lung cancer cells by activation of MEK-MAPK. |
| Name | kaempferol |
|---|---|
| Synonym | More Synonyms |
| Description | Kaempferol inhibits estrogen receptor α expression in breast cancer cells and induces apoptosis in glioblastoma cells and lung cancer cells by activation of MEK-MAPK. |
|---|---|
| Related Catalog | |
| Target |
Estrogen receptor[1] |
| In Vitro | Kaempferol also has anti-inflammatory effects via inhibition of interleukin-4 and cyclo-oxygenase 2 expression by suppressing Src kinase and downregulating the NFκB pathway. Kaempferol is also effective in inhibiting angiogenesis and inducing apoptosis in ovarian cancer cells[1]. Kaempferol is a natural flavonoid that is widely distributed in fruits and vegetables, and prospective studies revealed that over decades, consumption of Kaempferol dramatically and significantly reduces the risk of ovarian cancer in American female nurses. After a 24-hour treatment, Kaempferol causes a significant and concentration-dependent inhibition of proliferation in all 3 ovarian cancer cells tested. This inhibition is observed at 40 μM or higher concentrations of treatment[2]. Kaempferol is a flavonoid which is abundant in a variety of plant derived food and leaves used in traditional medicines. Kaempferol significantly inhibits NADPH oxidase activity. Kaempferol decrease reactive oxygen species (ROS) by directly bound NADPH oxidase. Kaempferol prevents Ang II-induced sinus nodal cell death by lowering CAMKII oxidization[3].10-20 μM Kaempferol dose-dependently suppresses its release in sensitized RBL-2H3 cells. When 10-20 μM Kaempferol is supplemented to DNP-BSA-challenged RBL-2H3 cells for 15 min, the activation of Syk and PLCγ is highly attenuated. When ≥10 μM Kaempferol is added to DNP-BSA-challenged RBL-2H3 cells for 60 min, the COX2 induction is reduced[4]. |
| In Vivo | The COX2 induction is confirmed in the airways of BSA-challenged BALB/c mice. There is lack of COX2 in airways of untreated control mice observed. The BSA inhalation to mice led to enhanced COX2 induction (dark brown staining) in mouse airway, which is reversed by oral administration of Kaempferol. In BSA-challenged mice, there is a marked goblet cell hyperplasia and epithelial thickening observed. When 20 mg/kg Kaempferol is supplemented to BSA-challenged mice, the epithelial thickening completely disappeared[4]. |
| Kinase Assay | Right atria or sinus nodal cells are homogenized in lysis buffer consisting of (50 mM Tris-HCl pH 7.5, 100 mM KCl, 1 mM ethylenediamine tetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 0.5 mM Benzamidine, 20 mg/L Leupeptin, 20 mM sodium pyrophosphate, 50 mM NaF, and 50 mM sodium β-glycerophosphate), and total protein content is determined by the Bradford assay. Caspase-3 activity is determined by EnzChek Caspase-3 Assay Kit[3]. |
| Cell Assay | Ovarian cancer cells are seeded in 96-well plates at 2000 cells/well and incubated overnight before treatment with 0-160 μM Kaempferol for 24 hours in triplicates. The medium is removed, and the plates are freeze-thawed to lyse cells. Each well is added with 200 μL 1× CyQUANT cell lysis buffer containing 5x SYBR Green I and incubated at room temperature (RT) for 5 minutes. The reaction (50 μL) is transferred to PCR strip tubes and the fluorescent signal is measured at 90°C with a real-time Chromo4 PCR instrument. To ensure that cell proliferation assays are performed within a linear range of cell numbers, a standard curve is generated by seeding different amount of OVCAR-3 cells (based on counting with a hemacytometer) in a 96-well plate, and measuring genomic DNA abundance after overnight incubation. Three independent experiments are performed and data is pooled for statistical analysis[2]. |
| Animal Admin | Mice[4] Three-week-old male BALB/c mice are randomly assigned to the four treatment groups as follows (n=8 per group). (1) PBS-sensitized mice; (2) BSA-sensitized mice; (3) BSA-sensitized and 10 mg/kg Kaempferol-administered mice; and (4) BSA-sensitized and 20 mg/kg Kaempferol-administered mice. Mice are given a commercial mouse chow diet containing 20.5% protein, 3.5% fat, 8% fiber, 8% ash, and 0.5% phosphorus and are allowed access to food and water ad libitum. The mice are kept under a 12 h light and dark cycle at 23±1°C with 50%±5% relative humidity in specific pathogen-free conditions. Mice are allowed to become accustomed to their surroundings for one week before starting the allergic experiments. Sensitization of all experimental mice is carried out by subcutaneous injection with 20 μg BSA in 30 μL PBS and 50 μL Imject Alum on days 0 and 14. The control mice are injected with a combination of 50 μL PBS and 50 μL Imject Alum without BSA. On days 28, 29, and 30, only the experimental mice sensitized to BSA are subject to inhalation of 5% BSA, while control mice are challenged with 5% PBS for 20 min in a plastic chamber connected to a Medel aerosol nebulizer. All mice are sacrificed 24 h after the last challenge. Whole blood samples are directly used to measure the contents of eosinophils, basophils and neutrophils. The right lung is stored in 4% paraformaldehyde until use. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 582.1±50.0 °C at 760 mmHg |
| Melting Point | 276°C |
| Molecular Formula | C15H10O6 |
| Molecular Weight | 286.236 |
| Flash Point | 226.1±23.6 °C |
| Exact Mass | 286.047729 |
| PSA | 111.13000 |
| LogP | 2.05 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.785 |
| InChIKey | IYRMWMYZSQPJKC-UHFFFAOYSA-N |
| SMILES | O=c1c(O)c(-c2ccc(O)cc2)oc2cc(O)cc(O)c12 |
| Storage condition | −20°C |
| Water Solubility | ethanol: 20 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H341 |
| Precautionary Statements | P281-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | UN 2811 6.1 / PGIII |
| WGK Germany | - |
| RTECS | LK9275200 |
| HS Code | 2914501900 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914501900 |
|---|---|
| Summary | 2914501900 other ketone-phenols。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Antiviral effect of methylated flavonol isorhamnetin against influenza.
PLoS ONE 10(3) , e0121610, (2015) Influenza is an infectious respiratory disease with frequent seasonal epidemics that causes a high rate of mortality and morbidity in humans, poultry, and animals. Influenza is a serious economic conc... |
|
|
Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts.
Biochem. Pharmacol. 96 , 337-48, (2015) During senescence, cells express molecules called senescence-associated secretory phenotype (SASP), including growth factors, proinflammatory cytokines, chemokines, and proteases. The SASP induces a c... |
|
|
Kaempferol-3-O-rutinoside from Afgekia mahidoliae promotes keratinocyte migration through FAK and Rac1 activation.
J. Nat. Med. 69 , 340-8, (2015) The restoration of the epidermal epithelium through re-epithelialization is a critical process in wound healing. Directed keratinocyte migration to the wound is required, and the retardation of this p... |
| 3,4',5,7-Tetrahydroxyflavone Hydrate |
| Pelargidenolon |
| EINECS 208-287-6 |
| campherol |
| 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one |
| KaeMpferol Hydrate |
| MFCD00016938 |
| Swartziol |
| asurone |
| 3,5,7,4'-tetrahydroxyflavone |
| nimbecetin |
| Trifolitin |
| Populnetin |
| 3 4' 5 7-tetrahydroxyflavone |
| 5,7,4'-trihydroxyflavonol |
| kampherol |
| Kaempferol |
| Rhamnolutin |
| Robigenin |
| Swartsiol |
| Rhamnolutein |
| 3,4',5,7-tetrahydroxyflavone |
| 3,4,5,7-Tetrahydroxyflavone |
| kempferol |
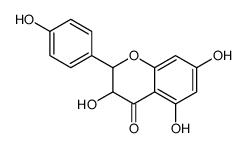 CAS#:724434-08-6
CAS#:724434-08-6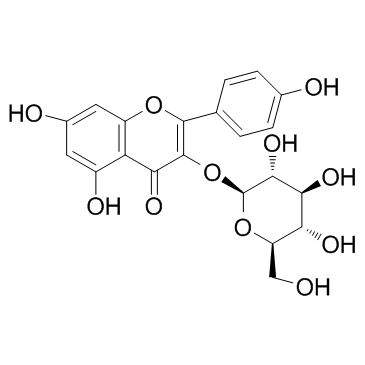 CAS#:480-10-4
CAS#:480-10-4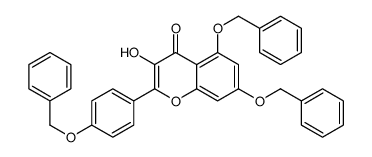 CAS#:23405-70-1
CAS#:23405-70-1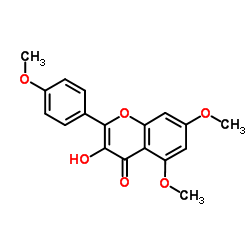 CAS#:1098-92-6
CAS#:1098-92-6 CAS#:480-20-6
CAS#:480-20-6 CAS#:482-39-3
CAS#:482-39-3 CAS#:22688-78-4
CAS#:22688-78-4 CAS#:1592-70-7
CAS#:1592-70-7 CAS#:491-54-3
CAS#:491-54-3 CAS#:487-70-7
CAS#:487-70-7 CAS#:99-96-7
CAS#:99-96-7 CAS#:482-38-2
CAS#:482-38-2 CAS#:90-18-6
CAS#:90-18-6![[4-(3,5,7-trihydroxy-4-oxochromen-2-yl)phenyl] hydrogen sulfate structure](https://image.chemsrc.com/caspic/338/62369-23-7.png) CAS#:62369-23-7
CAS#:62369-23-7 CAS#:108-73-6
CAS#:108-73-6 CAS#:51-28-5
CAS#:51-28-5
