Chloroquine
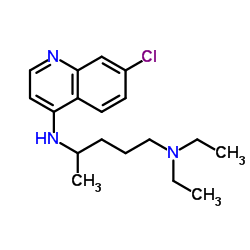
Chloroquine structure
|
Common Name | Chloroquine | ||
|---|---|---|---|---|
| CAS Number | 54-05-7 | Molecular Weight | 319.872 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 460.6±40.0 °C at 760 mmHg | |
| Molecular Formula | C18H26ClN3 | Melting Point | 87ºC | |
| MSDS | N/A | Flash Point | 232.3±27.3 °C | |
Use of ChloroquineChloroquine is an antimalarial and anti-inflammatory agent widely used to treat malaria and rheumatoid arthritis. Chloroquine is a autophagy and toll-like receptors (TLRs) inhibitor. Chloroquine is highly effective in the control of SARS-CoV-2 (COVID-19) infection in vitro (EC50=1.13 μM)[1][2][3][4]. |
| Name | chloroquine |
|---|---|
| Synonym | More Synonyms |
| Description | Chloroquine is an antimalarial and anti-inflammatory agent widely used to treat malaria and rheumatoid arthritis. Chloroquine is a autophagy and toll-like receptors (TLRs) inhibitor. Chloroquine is highly effective in the control of SARS-CoV-2 (COVID-19) infection in vitro (EC50=1.13 μM)[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
TLRs, HIV, SARS-CoV-2, Autophagy[1][2][3][4] |
| In Vitro | Chloroquine (CHQ, 20 μM) inhibits IL-12p70 release and reduces Th1-priming capacity of activated human monocyte-derived Langerhans-like cells (MoLC). Chloroquine (20 μM) enhances IL-1–induced IL-23 secretion in MoLC and subsequently increases IL-17A release by primed CD4+ T cells[1]. Chloroquine (25 μM) suppresses MMP-9 mRNA expression in normoxia and hypoxia in parental MDA-MB-231 cells. Chloroquine has cell-, dose- and hypoxia-dependent effects on MMP-2, MMP-9 and MMP-13 mRNA expression[2]. TLR7 and TLR9 inhibition using IRS-954 or chloroquine significantly reduces HuH7 cell proliferation in vitro[3]. Chloroquine (0.01-100μM; 48 hours) potently blocked virus infection (vero E6 cells infected with SARS-CoV-2) at low-micromolar concentration (EC50=1.13 μM). Chloroquine blocks virus infection by increasing endosomal pH required for virus/cell fusion, as well as interfering with the glycosylation of cellular receptors of SARS-CoV[4]. |
| In Vivo | Chloroquine (80 mg/kg, i.p.) does not prevent the growth of the triple-negative MDA-MB-231 cells with high or low TLR9 expression levels in the orthotopic mouse model[2]. TLR7 and TLR9 inhibition using IRS-954 or chloroquine significantly inhibits tumour growth in the mouse xenograft model. HCC development in the DEN/NMOR rat model is also significantly inhibited by Chloroquine[3]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 460.6±40.0 °C at 760 mmHg |
| Melting Point | 87ºC |
| Molecular Formula | C18H26ClN3 |
| Molecular Weight | 319.872 |
| Flash Point | 232.3±27.3 °C |
| Exact Mass | 319.181519 |
| PSA | 28.16000 |
| LogP | 4.69 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.592 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | Xi |
|---|---|
| HS Code | 2933499090 |
|
~76% 
Chloroquine CAS#:54-05-7 |
| Literature: Margolis, Brandon J.; Long, Kimberly A.; Laird, Dana L. T.; Ruble, J. Craig; Pulley, Shon R. Journal of Organic Chemistry, 2007 , vol. 72, # 6 p. 2232 - 2235 |
|
~% 
Chloroquine CAS#:54-05-7 |
| Literature: Kuter, David; Benjamin, Stefan J.; Egan, Timothy J. Journal of Inorganic Biochemistry, 2014 , vol. 133, p. 40 - 49 |
|
~% 
Chloroquine CAS#:54-05-7 |
| Literature: De; Byers; Krogstad Journal of Heterocyclic Chemistry, 1997 , vol. 34, # 1 p. 315 - 320 |
|
~% 
Chloroquine CAS#:54-05-7 |
| Literature: De; Byers; Krogstad Journal of Heterocyclic Chemistry, 1997 , vol. 34, # 1 p. 315 - 320 |
|
~% 
Chloroquine CAS#:54-05-7 |
| Literature: De; Byers; Krogstad Journal of Heterocyclic Chemistry, 1997 , vol. 34, # 1 p. 315 - 320 |
|
~% 
Chloroquine CAS#:54-05-7 |
| Literature: De; Byers; Krogstad Journal of Heterocyclic Chemistry, 1997 , vol. 34, # 1 p. 315 - 320 |
|
~% 
Chloroquine CAS#:54-05-7 |
| Literature: De; Byers; Krogstad Journal of Heterocyclic Chemistry, 1997 , vol. 34, # 1 p. 315 - 320 |
|
~% 
Chloroquine CAS#:54-05-7 |
| Literature: Constantinidis, I.; Satterlee, James D. Journal of the American Chemical Society, 1988 , vol. 110, # 13 p. 4391 - 4395 |
|
~% 
Chloroquine CAS#:54-05-7 |
| Literature: De; Byers; Krogstad Journal of Heterocyclic Chemistry, 1997 , vol. 34, # 1 p. 315 - 320 |
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| UNII:886U3H6UFF |
| Bemaco |
| (±)-Chloroquine |
| N-(7-Chloro-4-quinolinyl)-N,N-diethyl-1,4-pentanediamine |
| [3H]-Chloroquine |
| sn7618 |
| N-(7-Chloroquinolin-4-yl)-N,N-diethylpentane-1,4-diamine |
| CHLOROQUINE |
| 1,4-Pentanediamine, N-(7-chloro-4-quinolinyl)-N,N-diethyl- |
| 1,4-Pentanediamine, N4-(7-chloro-4-quinolinyl)-N1,N1-diethyl- |
| N4-(7-Chloroquinolin-4-yl)-N1,N1-diethylpentane-1,4-diamine |
| Amokin |
| rp3377 |
| N4-(7-chloroquinoline-4-yl)-N1,N1-diethyl-pentane-1,4-diamine |
| Imagon |
| Aralen |
| w7618 |
| 3377 RP |
| win244 |
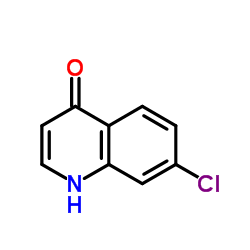
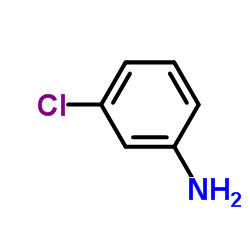
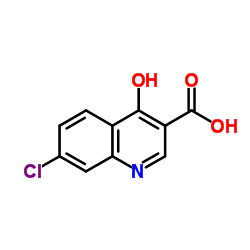
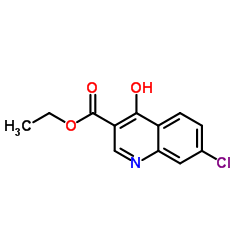
![Propanedioic acid,2-[[(3-chlorophenyl)amino]methylene]-, 1,3-diethyl ester structure](https://image.chemsrc.com/caspic/023/3412-99-5.png)
![3-[7,12,17-tris-(2-carboxy-ethyl)-3,8,13,18-tetrakis-carboxymethyl-22,24-dihydro-porphin-2-yl]-propionic acid structure](https://image.chemsrc.com/caspic/237/607-14-7.png)
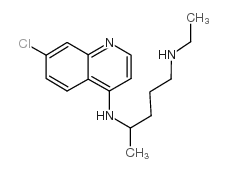 CAS#:1476-52-4
CAS#:1476-52-4
