CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
YS4550000
-
CHEMICAL NAME :
-
Urea, 1-butyl-3-(p-tolylsulfonyl)-
-
CAS REGISTRY NUMBER :
-
64-77-7
-
BEILSTEIN REFERENCE NO. :
-
1984428
-
LAST UPDATED :
-
199806
-
DATA ITEMS CITED :
-
40
-
MOLECULAR FORMULA :
-
C12-H18-N2-O3-S
-
MOLECULAR WEIGHT :
-
270.38
-
WISWESSER LINE NOTATION :
-
4MVMSWR D1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
1 gm/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting Endocrine - hypoglycemia
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2490 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
860 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
700 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
490 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
650 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - ataxia Endocrine - hypoglycemia Nutritional and Gross Metabolic - body temperature decrease
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
980 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
770 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
700 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Mammal - species unspecified
-
DOSE/DURATION :
-
750 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 1-13 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Effects on Newborn - other neonatal measures or effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2600 mg/kg
-
SEX/DURATION :
-
female 1-37 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system Reproductive - Effects on Newborn - other neonatal measures or effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
1800 mg/kg
-
SEX/DURATION :
-
female 26-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3 gm/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
12 gm/kg
-
SEX/DURATION :
-
female 1-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
8400 mg/kg
-
SEX/DURATION :
-
male 3 day(s) pre-mating female 3 day(s) pre-mating - 22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
70 mg/kg
-
SEX/DURATION :
-
female 7-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Effects on Newborn - live birth index (measured after birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
1650 mg/kg
-
SEX/DURATION :
-
female 1-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - biochemical and metabolic
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
5400 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1700 mg/kg
-
SEX/DURATION :
-
female 1-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1250 mg/kg
-
SEX/DURATION :
-
female 2 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
400 mg/kg
-
SEX/DURATION :
-
female 13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1800 mg/kg
-
SEX/DURATION :
-
female 8-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
Sister chromatid exchange
-
TYPE OF TEST :
-
Sister chromatid exchange
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Sister chromatid exchange
MUTATION DATA
-
TEST SYSTEM :
-
Rodent - hamster
-
DOSE/DURATION :
-
28600 ug/kg
-
REFERENCE :
-
MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 77,349,1980 *** REVIEWS *** TOXICOLOGY REVIEW KDYIA5 Kidney International. (Springer-Verlag New York, Inc., Service Center, 44 Hartz Way, Secaucus Secaucus, NJ 07094) V.1- 1972- Volume(issue)/page/year: 10,82,1976 TOXICOLOGY REVIEW INTEAG Internist. (Springer-Verlag New York, Inc., Service Center, 44 Hartz Way, Secaucus, NJ 07094) V.1- 1960- Volume(issue)/page/year: 15,7,1974 TOXICOLOGY REVIEW ARVPAX Annual Review of Pharmacology. (Palo Alto, CA) V.1-15, 1961-75. For publisher information, see ARPTDI. Volume(issue)/page/year: 5,447,1965 TOXICOLOGY REVIEW ADVPA3 Advances in Pharmacology. (New York, NY) V.1-6, 1962-68. For publisher information, see AVPCAQ. Volume(issue)/page/year: 4,263,1966 TOXICOLOGY REVIEW LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 2,1424,1960 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X4772 No. of Facilities: 66 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 857 (estimated) No. of Female Employees: 600 (estimated)
|
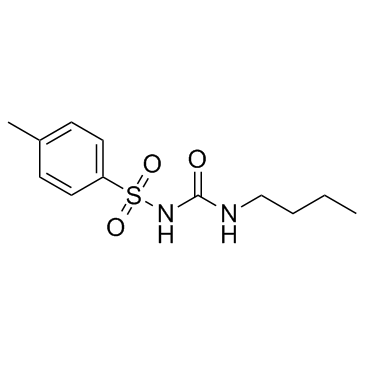
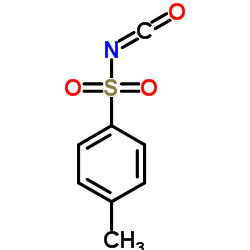 CAS#:4083-64-1
CAS#:4083-64-1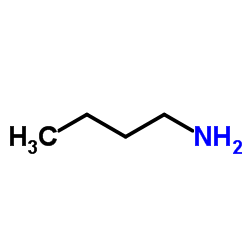 CAS#:109-73-9
CAS#:109-73-9 CAS#:39078-72-3
CAS#:39078-72-3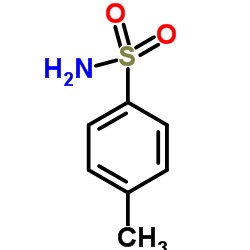 CAS#:70-55-3
CAS#:70-55-3 CAS#:39764-19-7
CAS#:39764-19-7 CAS#:27095-02-9
CAS#:27095-02-9 CAS#:111-36-4
CAS#:111-36-4 CAS#:4932-55-2
CAS#:4932-55-2 CAS#:1792-17-2
CAS#:1792-17-2 CAS#:18522-92-4
CAS#:18522-92-4 CAS#:592-31-4
CAS#:592-31-4 CAS#:613-33-2
CAS#:613-33-2 CAS#:108-88-3
CAS#:108-88-3 CAS#:5719-85-7
CAS#:5719-85-7 CAS#:1515-72-6
CAS#:1515-72-6 CAS#:36323-21-4
CAS#:36323-21-4 CAS#:3083-88-3
CAS#:3083-88-3 CAS#:1119-49-9
CAS#:1119-49-9 CAS#:1888-33-1
CAS#:1888-33-1
