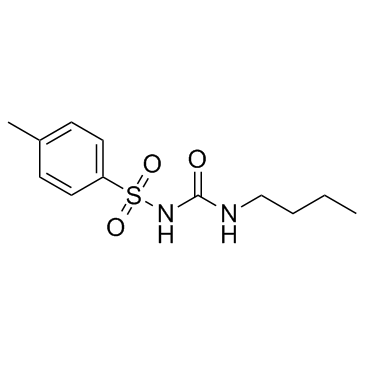
4 hydroxy tolbutamide

4 hydroxy tolbutamide structure
|
Common Name | 4 hydroxy tolbutamide | ||
|---|---|---|---|---|
| CAS Number | 5719-85-7 | Molecular Weight | 286.347 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C12H18N2O4S | Melting Point | 100-102ºC | |
| MSDS | Chinese USA | Flash Point | 2℃ | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of 4 hydroxy tolbutamide4-Hydroxytolbutamide (Hydroxytolbutamide) is a metabolite of Tolbutamide. 4-Hydroxytolbutamide is metabolized by CYP2C8 and CYP2C9. Tolbutamide is a first generation potassium channel blocker and a sulfonylurea oral antidiabetic[1][2]. |
| Name | 4-hydroxytolbutamide |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Hydroxytolbutamide (Hydroxytolbutamide) is a metabolite of Tolbutamide. 4-Hydroxytolbutamide is metabolized by CYP2C8 and CYP2C9. Tolbutamide is a first generation potassium channel blocker and a sulfonylurea oral antidiabetic[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Potassium channel |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Melting Point | 100-102ºC |
| Molecular Formula | C12H18N2O4S |
| Molecular Weight | 286.347 |
| Flash Point | 2℃ |
| Exact Mass | 286.098724 |
| PSA | 103.88000 |
| LogP | 0.70 |
| Index of Refraction | 1.553 |
| InChIKey | SJRHYONYKZIRPM-UHFFFAOYSA-N |
| SMILES | CCCCNC(=O)NS(=O)(=O)c1ccc(CO)cc1 |
| Storage condition | 2-8°C |
| Water Solubility | ethanol: 12 mg/mL |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H312 + H332-H319 |
| Precautionary Statements | P210-P261-P302 + P352 + P312-P304 + P340 + P312-P337 + P313-P403 + P235 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | F,Xn |
| Risk Phrases | 11-20/21/22-36 |
| Safety Phrases | 16-36/37-24/25 |
| RIDADR | UN 1648 3 / PGII |
| WGK Germany | 3 |
| HS Code | 29350090 |
|
~82% 
4 hydroxy tolbu... CAS#:5719-85-7 |
| Literature: Makaya; Irie; Shibasaki Chemical and Pharmaceutical Bulletin, 1983 , vol. 31, # 7 p. 2518 - 2519 |
|
~% 
4 hydroxy tolbu... CAS#:5719-85-7 |
| Literature: Xenobiotica, , vol. 25, # 12 p. 1345 - 1354 |
|
~% 
4 hydroxy tolbu... CAS#:5719-85-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 31, # 7 p. 2518 - 2519 |
|
~% 
4 hydroxy tolbu... CAS#:5719-85-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 31, # 7 p. 2518 - 2519 |
|
The in-vitro effect of complementary and alternative medicines on cytochrome P450 2C9 activity.
J. Pharm. Pharmacol. 66(9) , 1339-46, (2014) The aim of this study is to establish the inhibitory effects of 14 commonly used complementary and alternative medicines (CAM) on the metabolism of cytochrome P450 2C9 (CYP2C9) substrates 7-methoxy-4-... |
|
|
Metabolic characterization of meso-dihydroguaiaretic acid in liver microsomes and in mice.
Food Chem. Toxicol. 76 , 94-102, (2015) meso-Dihydroguaiaretic acid (MDGA) is a major component of Myristica fragrans and Machilus thunbergii that is traditionally used as a spice and for medicinal purposes. Despite reports of various biolo... |
|
|
Evaluation of thein vitro/in vivodrug interaction potential of BST204, a purified dry extract of ginseng, and its four bioactive ginsenosides through cytochrome P450 inhibition/induction and UDP-glucuronosyltransferase inhibition
Food Chem. Toxicol. 68 , 117-27, (2014) • BST204 is a purified dry extract of ginseng containing high amounts of Rh2 and Rg3. • BST204 had only weak inhibitory effects on nine CYPs and five UGTs. • It is unlikely that BST204 alter pharmacok... |
| 1-Butyl-3-(4-hydroxymethylphenyl)sulfonylurea |
| Benzenesulfonamide, N-[(butylamino)carbonyl]-4-(hydroxymethyl)- |
| hydroxymethyl-TB |
| 4-OH-tolbutamide |
| Methylhydroxytolbutamide |
| hydroxymethyltolbutamide |
| Hydroxytolbutamide |
| 4-Hydroxytolbutamide |
| 4 hydroxy tolbutamide |
| N-(Butylcarbamoyl)-4-(hydroxymethyl)benzenesulfonamide |
| [14C]-Hydroxytolbutamide |
| N-(Butylaminocarbonyl)-4-hydroxymethylbenzenesulfonamide |
| 1-butyl-3-[4-(hydroxymethyl)phenyl]sulfonylurea |


 CAS#:88241-95-6
CAS#:88241-95-6