Chlorpromazine hydrochloride
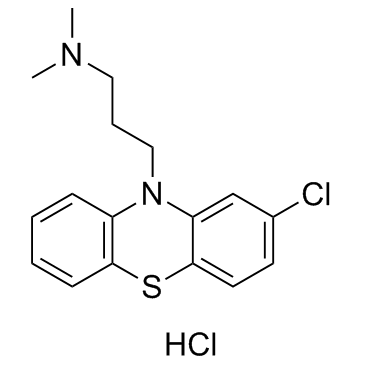
Chlorpromazine hydrochloride structure
|
Common Name | Chlorpromazine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 69-09-0 | Molecular Weight | 355.325 | |
| Density | 1.077 g/cm3 (15 C) | Boiling Point | 450.1ºC at 760 mmHg | |
| Molecular Formula | C17H20Cl2N2S | Melting Point | 192-196°C | |
| MSDS | Chinese USA | Flash Point | 48.2 °F | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Chlorpromazine hydrochlorideChlorpromazine Hydrochloride is an antagonist of the dopamine D2, 5HT2A, potassium channel andsodium channel. Chlorpromazine binds with D2 and 5HT2A with Kis of 363 nM and 8.3 nM, respectively. |
| Name | chlorpromazine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Chlorpromazine Hydrochloride is an antagonist of the dopamine D2, 5HT2A, potassium channel andsodium channel. Chlorpromazine binds with D2 and 5HT2A with Kis of 363 nM and 8.3 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
Ki: 363 nM (dopamine D2 receptor), 8.3 nM (5-HT2A receptor)[4] |
| In Vitro | Chlorpromazine (3, 10, 20, 40, and 60 μM) decreases the peak currents of hNav1.7 in a concentration-dependent manner, with IC50 of 25.9 μM with a Hill coefficient of 2.3. Chlorpromazine (25 μM) produces strong use-dependent inhibition of the hNav1.7 current. Chlorpromazine blocks the hNav1.7 channel, independent of calmodulin[1]. Chlorpromazine blocks HERG potassium channels with an IC50 value of 21.6 μM and a Hill coefficient of 1.11. Chlorpromazine (1, 10, 100 μM) blocks HERG potassium channels expressed in Xenopus laevis oocytes in a concentration-dependent manner. Chlorpromazine blocks HERG potassium channels in the activated state[5]. |
| In Vivo | Chlorpromazine (2 mg/kg, i.p.)-induced neurobehavioural abnormalities (NAs) are characterized by significant increase in cataleptic behaviour and loared spontaneous activity reaction time in mice[2]. Chlorpromazine (1 or 5 mg/kg, i,p.) prevents ketamine (KET) from increasing average spectral power of delta and gamma-high bands on the 5th and 10th days of treatment in rats[3]. |
| Animal Admin | Adult mice (8-10 weeks old) weighing 18-25 g are divided into five groups of six mice per group. The treatment schedule is as follows: Group 1, control (Normal Saline: NS, 10 mL/kg i.p.); Group 2, chlorpromazine (CPZ, 2 mg/kg i.p.); Group 3, bromocriptine (BMC, 2.5 mg/kg s.c.); Group 4: amlodipine (AML, 1 mg/kg s.c.); Group 5, BMC (2.5 mg/kg s.c.) + AML (1 mg/kg). Animal treated with BMC or AML or their combination also receive chlorpromazine 30 min later (i.p.). Animals are subjected to various tests including metal bar test for catalepsy and spontaneous activity wheel for motor assessment and agility and elevated plus maze, hole-board, Y-maze, open-field tests for locomotory activity, and exploratory behaviour respectively. Animals are euthanized eighteen hours later by cervical dislocation. The brain is dissected, rinsed in buffer (pH 7.6) and homogenized with Teflon and used for assessment of lipid peroxidation, reduced glutathione, superoxide dismutase and catalase. |
| References |
| Density | 1.077 g/cm3 (15 C) |
|---|---|
| Boiling Point | 450.1ºC at 760 mmHg |
| Melting Point | 192-196°C |
| Molecular Formula | C17H20Cl2N2S |
| Molecular Weight | 355.325 |
| Exact Mass | 354.072418 |
| PSA | 31.78000 |
| LogP | 5.76140 |
| Index of Refraction | 1.4436 (20ºC) |
| InChIKey | FBSMERQALIEGJT-UHFFFAOYSA-N |
| SMILES | CN(C)CCCN1c2ccccc2Sc2ccc(Cl)cc21.Cl |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. Air and light sesnsitive. |
| Water Solubility | >=10 g/100 mL at 24 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H330 |
| Precautionary Statements | P260-P284-P301 + P310-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+ |
| Risk Phrases | R25 |
| Safety Phrases | S28-S36/37-S45 |
| RIDADR | UN 2811 6.1/PG 1 |
| WGK Germany | 3 |
| RTECS | SO1750000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2932999099 |
| Flash Point(F) | 48.2 °F |
| Flash Point(C) | 9 °C |
|
~83% 
Chlorpromazine ... CAS#:69-09-0 |
| Literature: Galons; Miocque; Combet-Farnoux; et al. Chemical and Pharmaceutical Bulletin, 1985 , vol. 33, # 11 p. 5108 - 5109 |
| Precursor 2 | |
|---|---|
| DownStream 9 | |
| HS Code | 2934300000 |
|---|---|
| Summary | 2934300000. other compounds containing in the structure a phenothiazine ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells.
Nat. Commun. 6 , 6240, (2015) Epstein-Barr virus (EBV) is implicated as an aetiological factor in B lymphomas and nasopharyngeal carcinoma. The mechanisms of cell-free EBV infection of nasopharyngeal epithelial cells remain elusiv... |
|
|
Direct cellular delivery of human proteasomes to delay tau aggregation.
Nat. Commun. 5 , 5633, (2014) The 26S proteasome is the primary machinery that degrades ubiquitin (Ub)-conjugated proteins, including many proteotoxic proteins implicated in neurodegeneraton. It has been suggested that the elevati... |
|
|
Cargoing P-gp inhibitors via nanoparticle sensitizes tumor cells against doxorubicin.
Int. J. Pharm. 478(2) , 745-52, (2015) Inhibitors against multidrug resistance (MDR) efflux transporters have failed in most clinical settings due to unfavorable pharmacokinetic interactions with co-administered anti-cancer drug and their ... |
| CHLORAZIN |
| CHLORPROMAZINE HCL |
| chloracetil |
| Hibernal |
| Phenothiazine, 2-chloro-10-(3-dimethylaminopropyl)-, hydrochloride |
| 2-Chloro-10-[3-(dimethylamino)-1-propyl]phenothiazine Hydrochloride |
| Chloropromazin hydrochloride |
| Phenothiazine, 2-chloro-10-[3- (dimethylamino)propyl]-, monohydrochloride |
| Fenactil monohydrochloride |
| Ampliactil |
| hibanil |
| UNII-9WP59609J6 |
| Plegomazin |
| Largactil |
| 10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl-, hydrochloride (1:1) |
| Contomin hydrochloride |
| 3-(2-Chloro-10H-phenothiazin-10-yl)-N,N-dimethylpropan-1-amine hydrochloride (1:1) |
| Aminazin Hydrochloride |
| Propaphenin |
| Largaktyl |
| Chlorpromazine hydrochloride |
| Thorazine |
| Chloractil |
| Megaphen |
| MFCD00012654 |
| Chlorpromazine monohydrochloride |
| EINECS 200-701-3 |
| 2-Chloro-10-(3-dimethylaminopropyl)phenothiazine monohydrochloride |
| Propaphen |
| CHLOROPROMAZINE HYDROCHLORIDE |
| Taroctyl |
| 3-(2-Chloro-10H-phenothiazin-10-yl)-N,N-dimethyl-1-propanamine hydrochloride (1:1) |
| Chlorpromazine (hydrochloride) |

 CAS#:50-53-3
CAS#:50-53-3![10-[3-(dimethylamino)propyl]phenothiazin-2-ol structure](https://image.chemsrc.com/caspic/213/3926-64-5.png) CAS#:3926-64-5
CAS#:3926-64-5 CAS#:58-40-2
CAS#:58-40-2 CAS#:61-01-8
CAS#:61-01-8 CAS#:53-60-1
CAS#:53-60-1 CAS#:1156505-40-6
CAS#:1156505-40-6 CAS#:1156505-41-7
CAS#:1156505-41-7 CAS#:316-07-4
CAS#:316-07-4 CAS#:969-99-3
CAS#:969-99-3
