ICI 118,551 (hydrochloride)
Modify Date: 2024-01-07 00:18:09
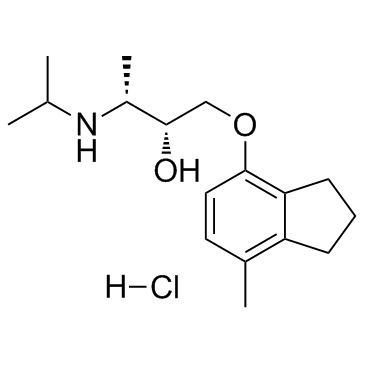
ICI 118,551 (hydrochloride) structure
|
Common Name | ICI 118,551 (hydrochloride) | ||
|---|---|---|---|---|
| CAS Number | 72795-01-8 | Molecular Weight | 313.863 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C17H28ClNO2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of ICI 118,551 (hydrochloride)ICI 118,551 (hydrochloride) is a highly selective β2 adrenergic receptor antagonist, with Ki values of 0.7, 49.5 and 611 nM for β2, β1 and β3 receptors, respectively. |
| Name | (2R,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | ICI 118,551 (hydrochloride) is a highly selective β2 adrenergic receptor antagonist, with Ki values of 0.7, 49.5 and 611 nM for β2, β1 and β3 receptors, respectively. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.7nM (β2 receptor), 49.5 nM (β1 receptor), 611 nM (β3 receptor) |
| In Vitro | ICI 118551 inhibits cAMP accumulation with IC50 of 1.7 μM in IMCD cells[1]. ICI 118551 (10 μM) induces a prominent vasorelaxation of norepinephrine (NE)-precontracted PA but not AO[2]. In failing human heart, ICI 118551 has significant effects on beat duration, with time-to-peak contraction and time-to-90% relaxation reduced compared with basal contraction. Negative Inotropic Effect of ICI 118551 Is Not cAMP-Related. Overexpression of β2AR in rabbit myocytes enhances negative inotropic effects of ICI 118551[3]. |
| In Vivo | ICI 118551 (0.2 mg/kg) injected into the jugular vein of the mice, reduces systolic pressure in the pulmonary circuit but not systemic arterial pressure[2]. |
| Kinase Assay | One hour prior to assay, the growth media are removed from the wells and replaced with 50 uL of Hanks’balanced salt solution that also contained 0.5 mM of MgCl2•6H2O, 0.4 mM of MgSO4•7H2O, 20 mM of N-2-hydroxyethylpiperazine-N’-2ethanesulfonic acid (HEPES), 1.2 mM of 3-isobutyl-1-methylxanthine (IBMX), 0.95 mM of CaCl2, and 0.05% of BSA. Each plate is placed in a 37°C shaking water bath for dose-response studies. In one study, various doses of isoproterenol (10-9-10-5 M) and β1- and β2-receptor-selective partial agonists (tazolol, prenalterol, salbutamol, and terbutaline, 10-6 and 10-5 M, respectively) are added (5 wells/dose/plate) and incubated for 10 min. In another study, the cells are stimulated with 10 μM isoproterenol in the presence or absence of various doses of β-adrenoceptor antagonists. The incubations are terminated after 10 min by the addition of 100 μL of 10% trichloroacetic acid (TCA) (final TCA concentration of 5%). TCA is removed twice by extraction with H20-saturated ether, and samples are dried at 80°C overnight, prior to resuspension in 50 mM of sodium acetate buffer. The CAMP content is measured with a radioimmunoassay kit. |
| References |
| Molecular Formula | C17H28ClNO2 |
|---|---|
| Molecular Weight | 313.863 |
| Exact Mass | 313.180847 |
| PSA | 41.49000 |
| LogP | 3.80280 |
| Storage condition | 2-8℃ |
| 2-Butanol, 1-[(2,3-dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-, (2S,3S)-, hydrochloride (1:1) |
| (2S,3S)-3-(Isopropylamino)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-2-butanol hydrochloride (1:1) |
| 3-(Isopropylamino)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-2-butanol hydrochloride (1:1) |
| ICI 118,551 hydrochloride |
| 2-Butanol, 1-[(2,3-dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-, hydrochloride (1:1) |
| 3-(Isopropylamino)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]butan-2-ol hydrochloride (1:1) |
| ICI 118,551 (hydrochloride) |