CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
CY2450000
-
CHEMICAL NAME :
-
Benzene, 4-allyl-1,2-dimethoxy-
-
CAS REGISTRY NUMBER :
-
93-15-2
-
BEILSTEIN REFERENCE NO. :
-
1910871
-
LAST UPDATED :
-
199712
-
DATA ITEMS CITED :
-
16
-
MOLECULAR FORMULA :
-
C11-H14-O2
-
MOLECULAR WEIGHT :
-
178.25
-
WISWESSER LINE NOTATION :
-
1U2R CO1 DO1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
810 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LC50 - Lethal concentration, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Inhalation
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>4800 mg/m3
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
540 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Behavioral - somnolence (general depressed activity) Behavioral - altered sleep time (including change in righting reflex)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
112 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Behavioral - somnolence (general depressed activity) Behavioral - altered sleep time (including change in righting reflex)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
>2025 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4200 mg/kg/6W-I
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
Unscheduled DNA synthesis
MUTATION DATA
-
TEST SYSTEM :
-
Rodent - mouse
-
DOSE/DURATION :
-
80 mg/kg
-
REFERENCE :
-
CRNGDP Carcinogenesis (London). (Oxford Univ. Press, Pinkhill House, Southfield Road, Eynsham, Oxford OX8 1JJ, UK) V.1- 1980- Volume(issue)/page/year: 5,1613,1984 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 04500 No. of Facilities: 41 (estimated) No. of Industries: 2 No. of Occupations: 21 No. of Employees: 2840 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 04500 No. of Facilities: 1074 (estimated) No. of Industries: 5 No. of Occupations: 10 No. of Employees: 12682 (estimated) No. of Female Employees: 9413 (estimated)
|







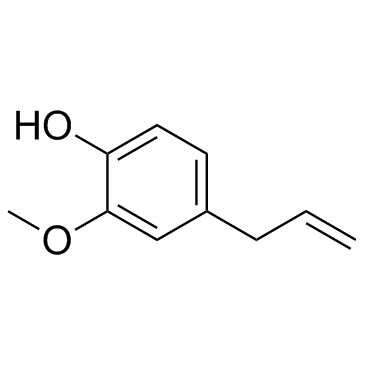 CAS#:97-53-0
CAS#:97-53-0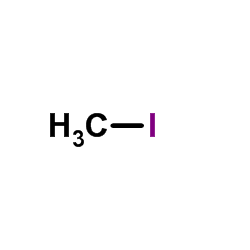 CAS#:74-88-4
CAS#:74-88-4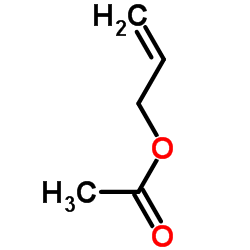 CAS#:591-87-7
CAS#:591-87-7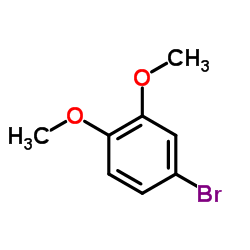 CAS#:2859-78-1
CAS#:2859-78-1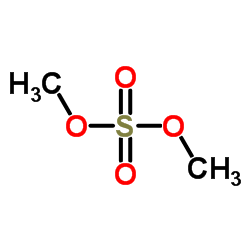 CAS#:77-78-1
CAS#:77-78-1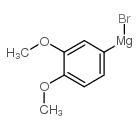 CAS#:89980-69-8
CAS#:89980-69-8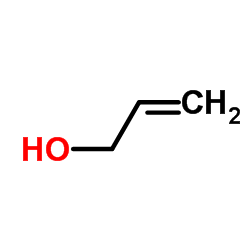 CAS#:107-18-6
CAS#:107-18-6 CAS#:627-40-7
CAS#:627-40-7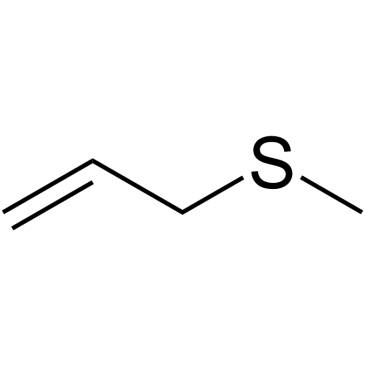 CAS#:10152-76-8
CAS#:10152-76-8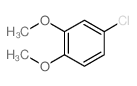 CAS#:16766-27-1
CAS#:16766-27-1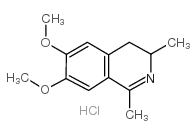 CAS#:107416-41-1
CAS#:107416-41-1 CAS#:3943-77-9
CAS#:3943-77-9 CAS#:5932-68-3
CAS#:5932-68-3![2-Methoxy-5-[(E)-1-propenyl]phenol structure](https://image.chemsrc.com/caspic/206/19784-98-6.png) CAS#:19784-98-6
CAS#:19784-98-6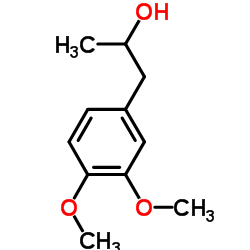 CAS#:19578-92-8
CAS#:19578-92-8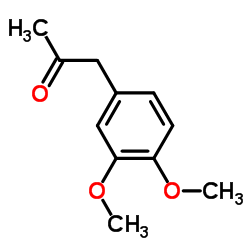 CAS#:776-99-8
CAS#:776-99-8 CAS#:120-14-9
CAS#:120-14-9 CAS#:5703-21-9
CAS#:5703-21-9 CAS#:27602-80-8
CAS#:27602-80-8 CAS#:93-40-3
CAS#:93-40-3
