2'-Deoxycytidine monohydrate
2'-Deoxycytidine monohydrate structure
|
Common Name | 2'-Deoxycytidine monohydrate | ||
|---|---|---|---|---|
| CAS Number | 951-77-9 | Molecular Weight | 227.22 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 482.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C9H13N3O4 | Melting Point | 209-211 °C(lit.) | |
| MSDS | USA | Flash Point | 245.4±31.5 °C | |
Use of 2'-Deoxycytidine monohydrate2'-Deoxycytidine, a deoxyribonucleoside, could inhibit biological effects of Bromodeoxyuridine (Brdu). |
| Name | 2'-deoxycytidine |
|---|---|
| Synonym | More Synonyms |
| Description | 2'-Deoxycytidine, a deoxyribonucleoside, could inhibit biological effects of Bromodeoxyuridine (Brdu). |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite Brdu |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 482.1±55.0 °C at 760 mmHg |
| Melting Point | 209-211 °C(lit.) |
| Molecular Formula | C9H13N3O4 |
| Molecular Weight | 227.22 |
| Flash Point | 245.4±31.5 °C |
| PSA | 119.83000 |
| LogP | -1.73 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.720 |
| InChIKey | CKTSBUTUHBMZGZ-SHYZEUOFSA-N |
| SMILES | Nc1ccn(C2CC(O)C(CO)O2)c(=O)n1 |
| Storage condition | Store at RT. |
| Water Solubility | H2O: 50 mg/mL, clear, colorless |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Precursor 8 | |
|---|---|
| DownStream 10 | |
|
Quantitation of endogenous nucleoside triphosphates and nucleosides in human cells by liquid chromatography tandem mass spectrometry.
Anal. Bioanal. Chem 407(13) , 3693-704, (2015) Nucleosides and nucleoside triphosphates are the building blocks of nucleic acids and important bioactive metabolites, existing in all living cells. In the present study, two liquid chromatography tan... |
|
|
Simultaneous quantification of purine and pyrimidine bases, nucleosides and their degradation products in bovine blood plasma by high performance liquid chromatography tandem mass spectrometry.
J. Chromatogr. A. 1356 , 197-210, (2014) Improved nitrogen utilization in cattle is important in order to secure a sustainable cattle production. As purines and pyrimidines (PP) constitute an appreciable part of rumen nitrogen, an improved u... |
|
|
Kinetic parameters and recognition of thymidine analogues with varying functional groups by thymidine phosphorylase.
Bioorg. Med. Chem. 16 , 3866-70, (2008) Thymidine phosphorylase (TP, EC 2.4.2.4) recognized the structure of the substrate with high specificity, via both the base and the ribosyl moieties. The replacement of 3'-OH of thymidine markedly inf... |
| 1-(2-Deoxy-β-D-ribofuranosyl)cytosine |
| EINECS 213-454-1 |
| 4-Amino-1-(2-deoxy-β-D-erythro-pentofuranosyl)-2(1H)-pyrimidinone |
| 4-Amino-1-(2-deoxy-β-D-glycero-pentofuranosyl)pyrimidin-2(1H)-one |
| 2-pyrimidinol, 1-(2-deoxy-β-D-erythro-pentofuranosyl)-1,4-dihydro-4-imino- |
| Cytidine, 2'-deoxy- |
| 1-(2-Deoxy-β-D-erythro-pentofuranosyl)-4-imino-1,4-dihydro-2-pyrimidinol |
| 1-(2-Deoxy-b-D-ribofuranosyl)cytosine |
| deoxycytidine |
| 2'-Deoxycytidine monohydrate |
| 4-Amino-1-(2-deoxy-b-D-erythro-pentofuranosyl)-2(1H)-pyrimidinone |
| 2(1H)-pyrimidinone, 4-amino-1-(2-deoxy-β-D-glycero-pentofuranosyl)- |
| 2‘-Deoxycytidine |
| 1-(2-deoxy-β-D-erythro-pentofuranosyl)-Cytosine |
| MFCD00006547 |
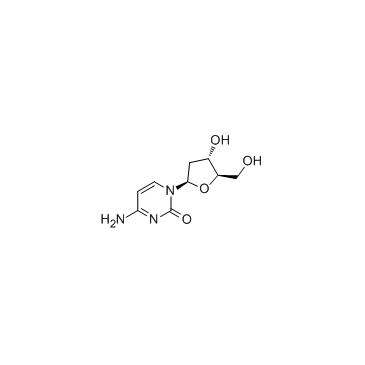
 CAS#:71-30-7
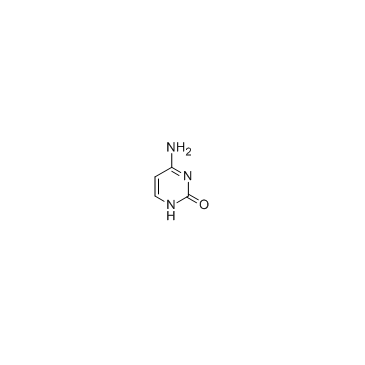
CAS#:71-30-7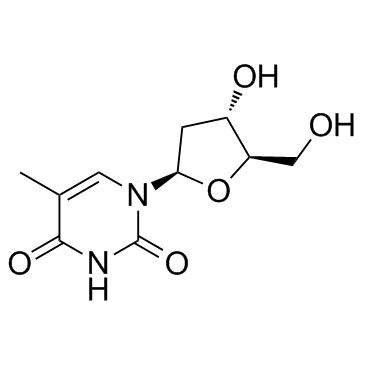 CAS#:50-89-5
CAS#:50-89-5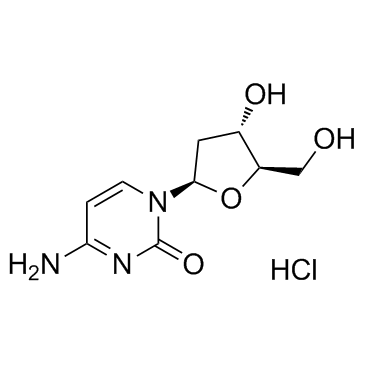 CAS#:3992-42-5
CAS#:3992-42-5 CAS#:380887-00-3
CAS#:380887-00-3 CAS#:7664-41-7
CAS#:7664-41-7 CAS#:108584-99-2
CAS#:108584-99-2 CAS#:78983-36-5
CAS#:78983-36-5 CAS#:65919-98-4
CAS#:65919-98-4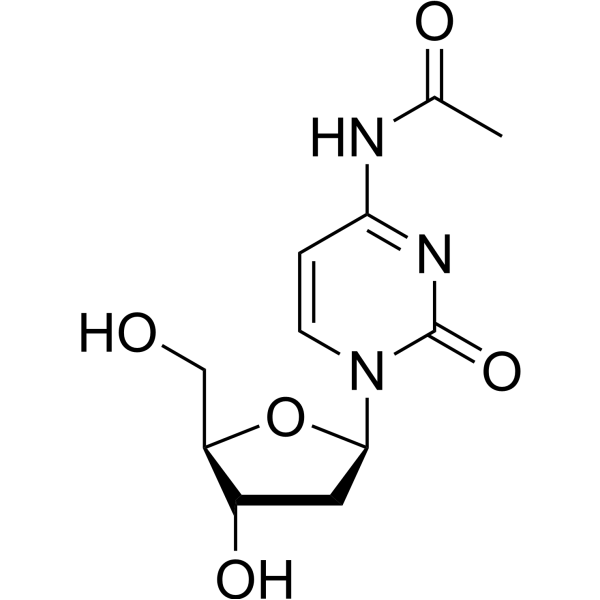 CAS#:32909-05-0
CAS#:32909-05-0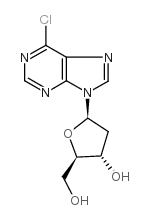 CAS#:4594-45-0
CAS#:4594-45-0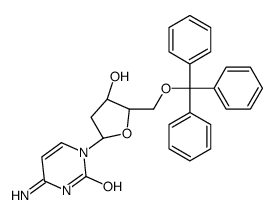 CAS#:18531-20-9
CAS#:18531-20-9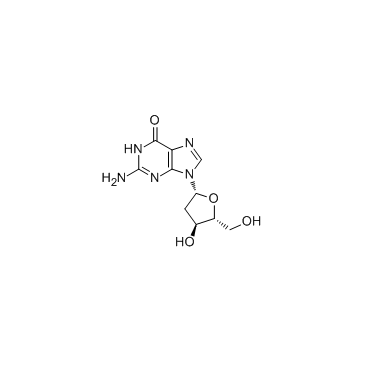 CAS#:961-07-9
CAS#:961-07-9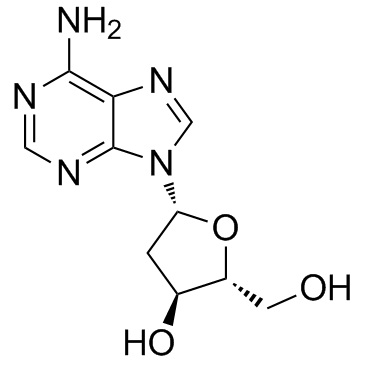 CAS#:958-09-8
CAS#:958-09-8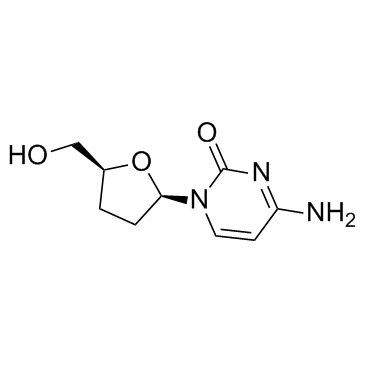 CAS#:7481-89-2
CAS#:7481-89-2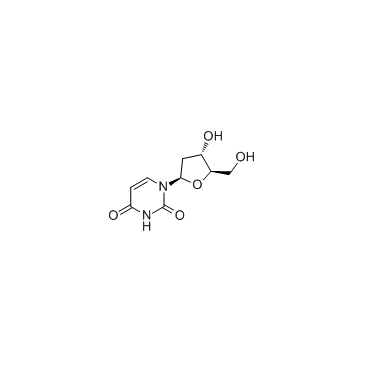 CAS#:951-78-0
CAS#:951-78-0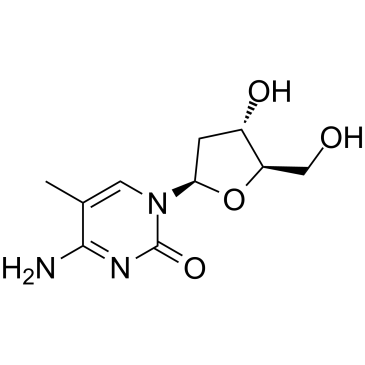 CAS#:838-07-3
CAS#:838-07-3 CAS#:67219-55-0
CAS#:67219-55-0
