2'-deoxyuridine
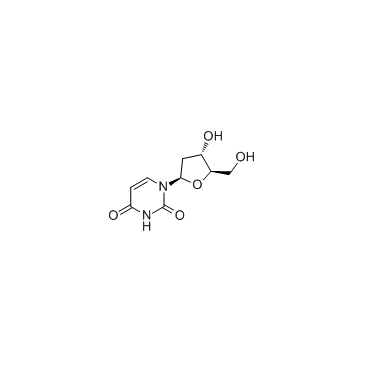
2'-deoxyuridine structure
|
Common Name | 2'-deoxyuridine | ||
|---|---|---|---|---|
| CAS Number | 951-78-0 | Molecular Weight | 228.202 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C9H12N2O5 | Melting Point | 167-169 °C(lit.) | |
| MSDS | USA | Flash Point | N/A | |
Use of 2'-deoxyuridine2'-Deoxyuridine could increase chromosome breakage and results in a decreased thymidylate synthetase activity. A known use of 2'-Deoxyuridine is as a precursor in the synthesis of Edoxudine. |
| Name | 2'-deoxyuridine |
|---|---|
| Synonym | More Synonyms |
| Description | 2'-Deoxyuridine could increase chromosome breakage and results in a decreased thymidylate synthetase activity. A known use of 2'-Deoxyuridine is as a precursor in the synthesis of Edoxudine. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Melting Point | 167-169 °C(lit.) |
| Molecular Formula | C9H12N2O5 |
| Molecular Weight | 228.202 |
| Exact Mass | 228.074615 |
| PSA | 104.55000 |
| LogP | -1.70 |
| Index of Refraction | 1.603 |
| InChIKey | MXHRCPNRJAMMIM-UHFFFAOYSA-N |
| SMILES | O=c1ccn(C2CC(O)C(CO)O2)c(=O)[nH]1 |
| Storage condition | 0-6°C |
| Water Solubility | 300 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | YU7490000 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
|
Simultaneous quantification of purine and pyrimidine bases, nucleosides and their degradation products in bovine blood plasma by high performance liquid chromatography tandem mass spectrometry.
J. Chromatogr. A. 1356 , 197-210, (2014) Improved nitrogen utilization in cattle is important in order to secure a sustainable cattle production. As purines and pyrimidines (PP) constitute an appreciable part of rumen nitrogen, an improved u... |
|
|
Phase I study of oral gemcitabine prodrug (LY2334737) in Japanese patients with advanced solid tumors.
Cancer Chemother. Pharmacol. 71(6) , 1645-55, (2013) LY2334737 is an oral gemcitabine prodrug. This Phase I study assessed the safety and tolerability of LY2334737 in Japanese patients with solid tumors and evaluated pharmacokinetics (PK), pharmacodynam... |
|
|
Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors.
J. Med. Chem. 51 , 6740-51, (2008) The work provides a new model for the prediction of the MAO-A and -B inhibitor activity by the use of combined complex networks and QSAR methodologies. On the basis of the obtained model, we prepared ... |
| Uracildeoxyr |
| deoxyuridine |
| 1-(2-Deoxy-β-D-erythro-pentofuranosyl)-4-hydroxy-2(1H)-pyrimidinone |
| T6NVMVJ A- ET5OTJ B1Q CQ &&Ribo-β-D Form |
| 2'-deoxy-uridin |
| 2,4(1H,3H)-Pyrimidinedione, 1-(2-deoxy-β-D-erythro-pentofuranosyl)- |
| 1-(2-Deoxy-b-D-erythro-pentofuranosyl)uracil |
| [131I]-Deoxyuridine |
| 2'-Deoxyuridine |
| Desoxyuridine |
| Uridine, 2'-deoxy- |
| MFCD00006527 |
| 2'-dU |
| EINECS 213-455-7 |
| 1-(2-Deoxy-D-erythro-pentofuranosyl)uracil |
| Deoxyribose uracil |
| 1-(2-deoxy-β-D-ribofuranosyl)-2,4(1H,3H)-Pyrimidinedione |
| Deoxy-uridin |
| 2′-Deoxyuridine |
| 2(1H)-Pyrimidinone, 1-(2-deoxy-β-D-erythro-pentofuranosyl)-4-hydroxy- |
| [3H]-Deoxyuridine |
| 1-(2-deoxy-β-D-erythro-pentofuranosyl)uracil |
| 1-(2-deoxy-β-δ-erythro-pentofuranosyl)-2,4(1H,3H)-Pyrimidinedione |
| 1-((2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione |
| durd |
| 2,4(1H,3H)-Pyrimidinedione, 1-(2-deoxy-β-D-ribofuranosyl)- |
| 1-[(2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-2,4(1H,3H)-pyrimidinedione |
| 1-(2-deoxy-β-δ-ribofuranosyl)-2,4(1H,3H)-Pyrimidinedione |
| DEOXYURIDINE-2' |
| 1-(2-deoxy-β-D-erythro-pentofuranosyl)-2,4(1H,3H)-Pyrimidinedione |
| 1-(2-Deoxy-β-D-erythro-pentofuranosyl)pyrimidin-2,4(1H,3H)-dion |
| 2'-DEOXYTHYMIDINE-5'-DIPHOSPHATE TRISODIUM SALT |
| 1-(2-Deoxy-δ-erythro-pentofuranosyl)uracil |
 CAS#:59-14-3
CAS#:59-14-3 CAS#:64911-18-8
CAS#:64911-18-8 CAS#:54-42-2
CAS#:54-42-2 CAS#:76700-78-2
CAS#:76700-78-2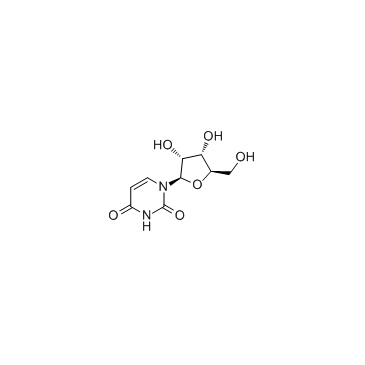 CAS#:58-96-8
CAS#:58-96-8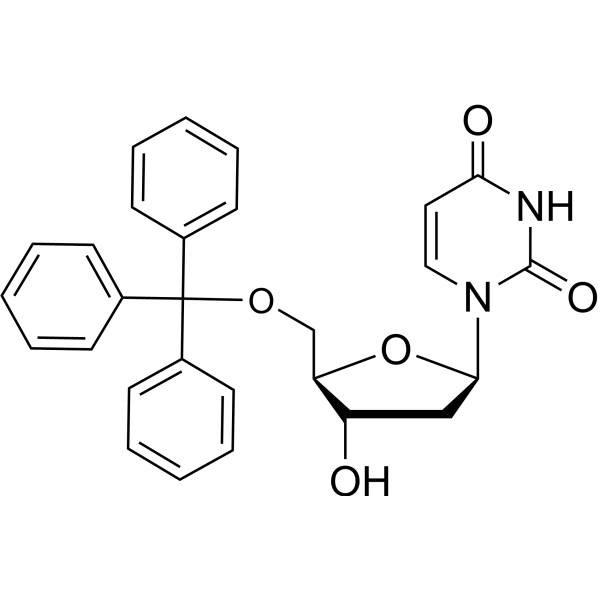 CAS#:14270-73-6
CAS#:14270-73-6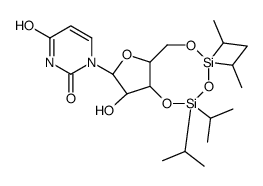 CAS#:69304-38-7
CAS#:69304-38-7![1-{4-hydroxy-5-[(4-methoxy-phenyl)-diphenyl-methoxymethyl]-tetrahydrofuran-2-yl}-1H-pyrimidine-2,4-dione Structure](https://image.chemsrc.com/caspic/060/70255-96-8.png) CAS#:70255-96-8
CAS#:70255-96-8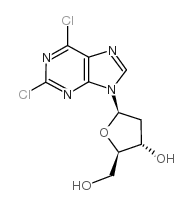 CAS#:37390-66-2
CAS#:37390-66-2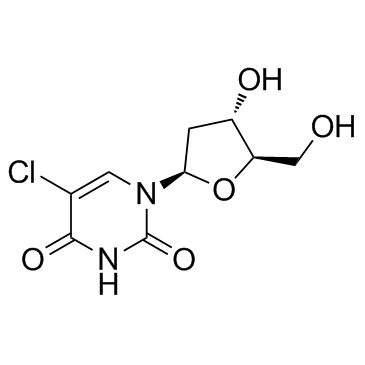 CAS#:50-90-8
CAS#:50-90-8 CAS#:66-22-8
CAS#:66-22-8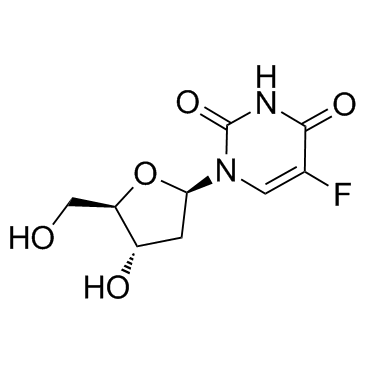 CAS#:50-91-9
CAS#:50-91-9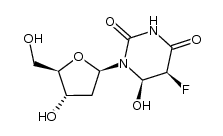 CAS#:3180-64-1
CAS#:3180-64-1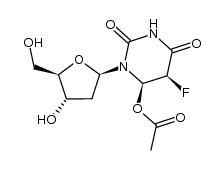 CAS#:119003-28-0
CAS#:119003-28-0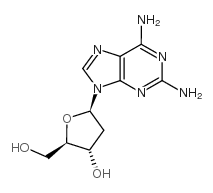 CAS#:4546-70-7
CAS#:4546-70-7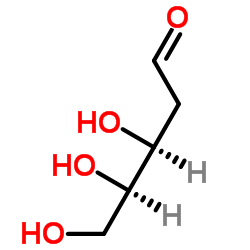 CAS#:533-67-5
CAS#:533-67-5
